| Image may be NSFW. Clik here to view. 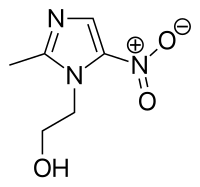 |
|
|---|---|
| Image may be NSFW. Clik here to view.  |
|
|
Metronidazole (INN) /(Flagyl, and others) is a nitroimidazoleantibiotic medication used particularly for anaerobic bacteria and protozoa. Metronidazole is an antibiotic, amebicide, and antiprotozoal. It is the drug of choice for first episodes of mild-to-moderate Clostridium difficile infection. It is marketed in the U.S.A. by Pfizer and globally by Sanofi under the trade name Flagyl, and is also sold under other brand names. Metronidazole was developed in 1960. Metronidazole is used also as a gel preparation in the treatment of the dermatologicalconditions such as rosacea (Rozex and MetroGel by Galderma) and fungating tumours(Anabact, Cambridge Healthcare Supplies). |
Image may be NSFW.
Clik here to view.
Synthesis
2-Methylimidazole (1) may be prepared via the Debus-Radziszewski imidazole synthesis, or from ethylenediamine and acetic acid, followed by treatment with lime, then Raney nickel. 2-Methylimidazole nitrated to give 2-methyl-4(5)-nitroimidazole (2), which is in turnalkylated with ethylene oxide or 2-chloroethanol to give metronidazole (3):[27][28][29]
Image may be NSFW.
Clik here to view.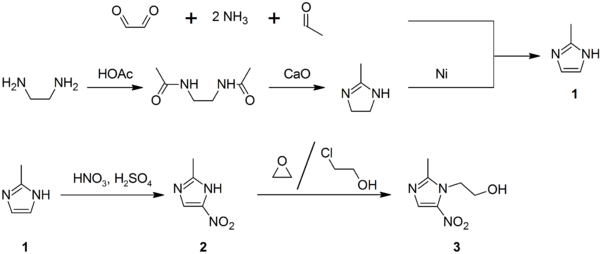
27- Ebel, K.; Koehler, H.; Gamer, A. O.; Jäckh, R. (2005), “Imidazole and Derivatives”, Ullmann’s Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH,doi:10.1002/14356007.a13_661
28-Actor, P.; Chow, A. W.; Dutko, F. J.; McKinlay, M. A. (2005), “Chemotherapeutics”, Ullmann’s Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH,doi:10.1002/14356007.a06_173
29-Kraft, M. Ya.; Kochergin, P. M.; Tsyganova, A. M.; Shlikhunova, V. S. (1989). “Synthesis of metronidazole from ethylenediamine”. Pharmaceutical Chemistry Journal 23 (10): 861–863. doi:10.1007/BF00764821.
Image may be NSFW.
Clik here to view.
- MORE
- Metronidazole (CAS NO.: 443-48-1), with its chemical name of 2-Methyl-5-nitro-1H-Imidazole-1-ethanol, could be produced through the following several reaction routes.1). Preparation method one:
The title compound can also be obtained by alkylation, in different solvents, of 1-(acetoxymethyl)-2-methyl-4-nitroImidazole(I) with either ethylene sulfate (II) or with bis-(2-acetoxyethyl) sulfate (III) -generated from ethyleneglycol diacetate (IV) and either dimethyl sulfate or H2SO4 - followed by hydrolysis or alcoholysis treatment.Image may be NSFW.
Clik here to view.
2). Preparation method two:
2-Methylimidazole (I) is converted into the bisulfate salt, and then nitrated by means of a sulfonitric mixture in Ac2O to produce 2-methyl-4-nitroimidazole (II) . In a variant of this procedure, 2-methylimidazole (I) is nitrated by using a ferric nitrate-tonsyl adduct in several solvents. Imidazole (II) is then regioselectively alkylated with boiling 2-chloroethanol to produce the title compound. Alternatively, the alkylation of (II) has been reported by treatment with ethylene oxide (III) under acidic conditions.Image may be NSFW.
Clik here to view.
Image may be NSFW.
Clik here to view.

.jpg)
.jpg)
