Image may be NSFW.
Clik here to view.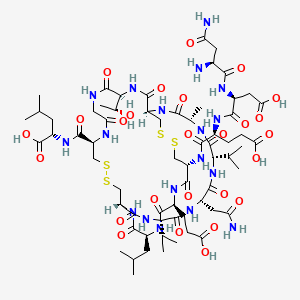
PLECANATIDE; UNII-7IK8Z952OK; (3-Glutamic acid(D>E))human uroguanylin (UGN); 467426-54-6;
| Molecular Formula: | C65H104N18O26S4 |
|---|---|
| Molecular Weight: | 1681.88626 g/mol |
IUPAC Condensed
H-Asn-Asp-Glu-Cys(1)-Glu-Leu-Cys(2)-Val-Asn-Val-Ala-Cys(1)-Thr-Gly-Cys(2)-Leu-OH
L-Leucine, L-asparaginyl-L-alpha-aspartyl-L-alpha-glutamyl-L-cysteinyl-L-alpha-glutamyl-L-leucyl-L- cysteinyl-L-valyl-L-asparaginyl-L-valyl-L-alanyl-L-cysteinyl-L-threonylglycyl-L-cysteinyl-, cyclic (4->12),(7->15)-bis(disulfide)
L-Asparaginyl-L-α-a
Plecanatide
RN: 467426-54-6
- Molecular FormulaC65H104N18O26S4
- Average mass1681.886 Da
Clik here to view.

Clik here to view.

Novel Chronic Idiopathic Constipation Drug Under FDA Review
Clik here to view.

Plecanatide is a once-daily, oral, uroguanylin analog
Synergy Pharmaceuticals announced the Food and Drug Administration (FDA) has accepted for review the New Drug Application (NDA) for plecanatide for the treatment of chronic idiopathic constipation (CIC).
The NDA submission was based on data from two double-blind, placebo-controlled Phase 3 trials and one open-label long term safety study in over 3,500 patients with CIC.
RELATED: NDA Submitted for Chronic Idiopathic Constipation Drug Plecanatide
The FDA has set a Prescription Drug User Fee Act (PDUFA) target action date of January 29, 2017 to make a decision on the NDA.
Plecanatide is a once-daily, oral, uroguanylin analog currently under development for the treatment of CIC and irritable bowel syndrome with constipation (IBS-C). It is designed to replicate the function of uroguanylin, a naturally occurring GI peptide, by working locally in the upper GI tract to stimulate digestive fluid movement and support regular bowel function.
///
C[C@H]1C(=O)

