Development of a concise, scalable synthesis of a CCR1 antagonist utilizing a continuous flow Curtius rearrangement
DOI: 10.1039/C6GC03123D, Paper
A convergent and robust synthesis of a developmental CCR1 antagonist is described using continuous flow technology
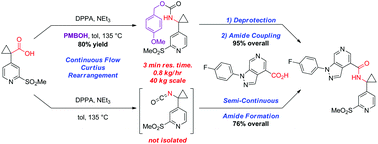
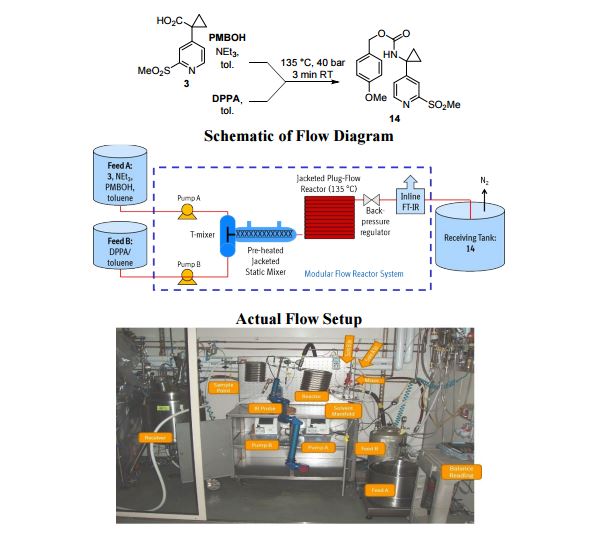
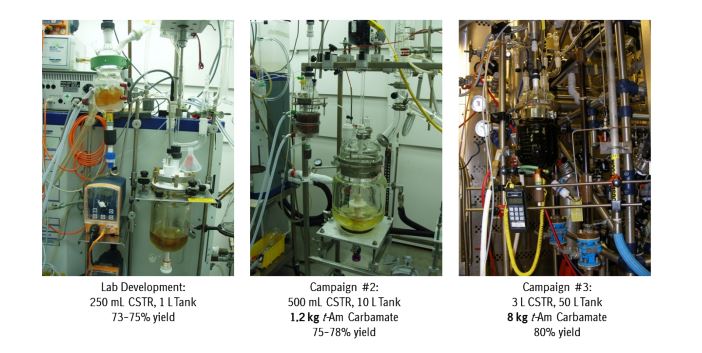
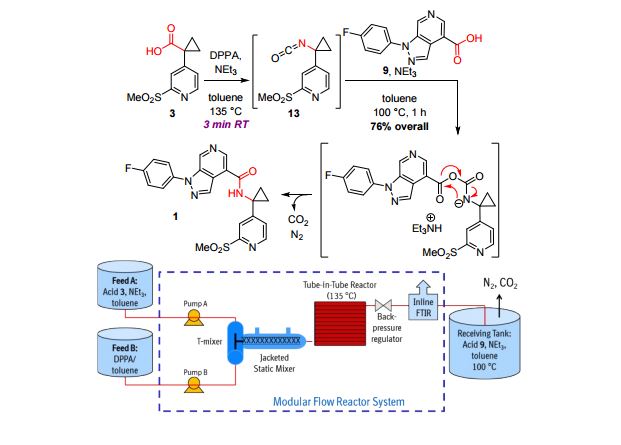
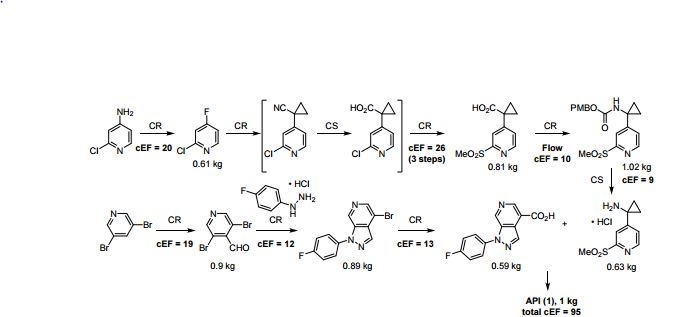
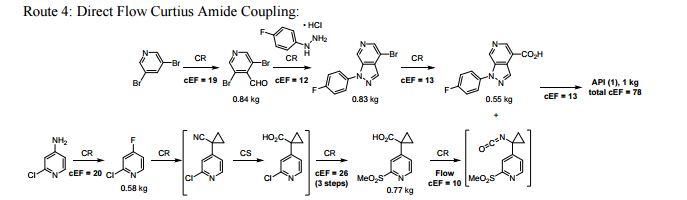
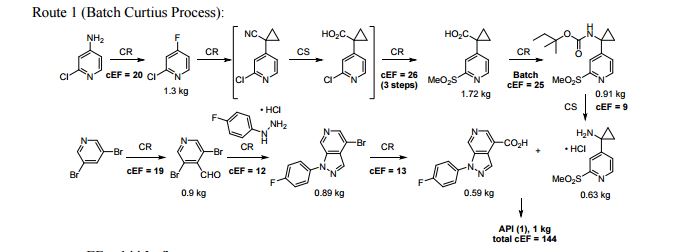
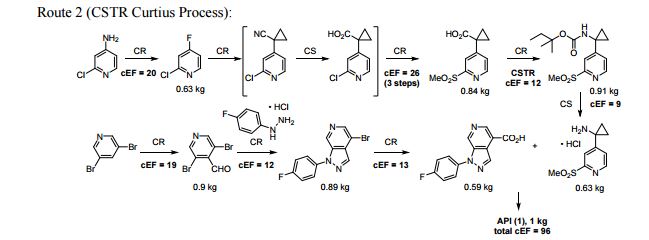
A convergent, robust, and concise synthesis of a developmental CCR1 antagonist is described using continuous flow technology. In the first approach, following an expeditious SNAr sequence for cyclopropane introduction, a safe, continuous flow Curtius rearrangement was developed for the synthesis of a p-methoxybenzyl (PMB) carbamate. Based on kinetic studies, a highly efficient and green process comprising three chemical transformations (azide formation, rearrangement, and isocyanate trapping) was developed with a relatively short residence time and high material throughput (0.8 kg h−1, complete E-factor = ∼9) and was successfully executed on 40 kg scale. Moreover, mechanistic studies enabled the execution of a semi-continuous, tandem Curtius rearrangement and acid–isocyanate coupling to directly afford the final drug candidate in a single, protecting group-free operation. The resulting API synthesis is further determined to be extremely green (RPG = 166%) relative to the industrial average for molecules of similar complexity.
Development of a concise, scalable synthesis of a CCR1 antagonist utilizing a continuous flow Curtius rearrangement
E-mail: maurice.marsini@boehringer-ingelheim.com
DOI: 10.1039/C6GC03123D
1-(4-fluorophenyl)-N-(1-(2-(methylsulfonyl)pyridin-4-yl)cyclopropyl)-1H-pyrazolo[3,4- c]pyridine-4-carboxamide
1-(4-fluorophenyl)-N-(1-(2-(methylsulfonyl)pyridin-4-yl)cyclopropyl)-1H-pyrazolo[3,4- c]pyridine-4-carboxamide
m.p. = 140-144 °C;
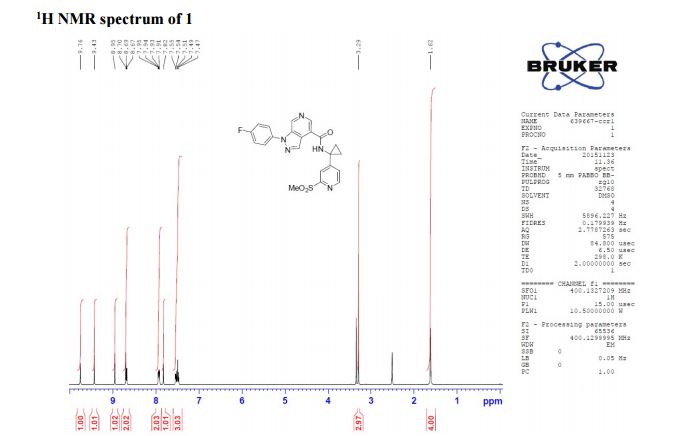
1H NMR (400 MHz, CDCl3) δ 9.76 (s, 1H), 9.43 (s, 1H), 8.95 (s, 1H), 8.70 (s, 1H), 8.68 (d, J = 5.2 Hz, 1H), 7.93 (s, J1 = 8.8 Hz, J2 = 4.7 Hz, 1H), 7.82 (s, 1H), 7.54 (d, J = 4.1 Hz, 1H), 7.49 (t, J = 8.7 Hz, 1H), 3.29 (s, 3H), 1.61 (bs, 4H);
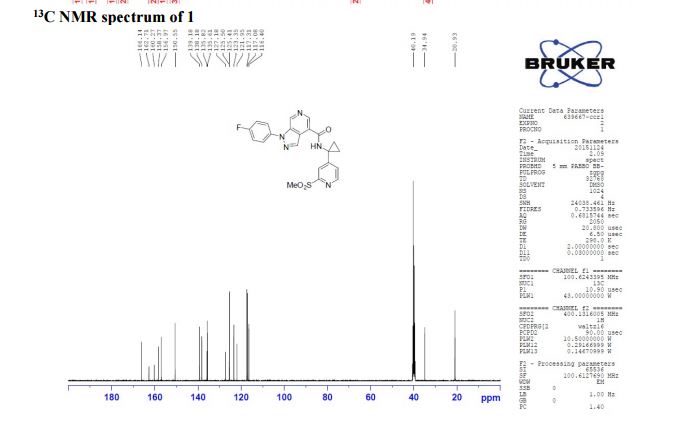
13C NMR (100 MHz, CDCl3) δ 166.1, 162.7, 160.3, 158.4, 156.9, 150.6, 139.2, 138.2, 135.8, 135.6, 125.4 (d, JC-F = 8.8 Hz), 123.3, 121.9, 117.2 (d, JC-F = 23.1 Hz), 116.4, 40.2, 34.9, 20.9;
HRMS: calcd for C22H19FN5O3S [M + H+ ]: 452.1187. Found: 452.1189.
//////////