GR-167089
ISV-611
………………………………

………………………………

 amcrasto@gmail.com
amcrasto@gmail.com
 amcrasto@gmail.com
amcrasto@gmail.com
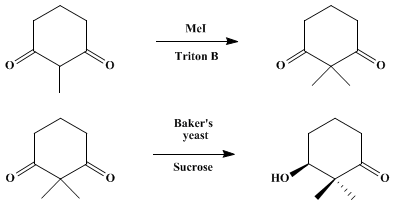
(S)-(+)-3-hydroxy-2,2-dimethylcyclohexanone
bp 85–87°C at 3.7 mm, [α]21D + 23.0° (CHCl3, c 2.0)
The spectral properties of (S)-(+)-3-hydroxy-2,2-dimethylcyclohexanone are as follows:
IR vmax (film) cm−1: 3470 (s), 1705 (s), 1120 (m), 1055 (s), 985 (s), 965 (m);
1H NMR (250 MHz, CDCl3) δ: 1.11 (s, 3 H), 1.15 (s, 3 H), 1.60–1.71 (m, 1 H), 1.76–1.86 (m, 1 H), 1.96–2.05 (m, 2 H), 2.16 (br s, 1 H), 2.35–2.45 (m, 2 H), 3.69 (dd, 1 H, J = 7.6, 2.9);
13C NMR (76 MHz, CDCl3) δ: 19.7, 20.7, 22.9, 29.0, 37.3, 51.3, 77.8, 215.3.
NOTE….Intermediate is
2,2-dimethylcyclohexane-1,3-dione bp 92–97°C (4 mm)
The spectra are as follows: 1H NMR (250 MHz, CDCl3) δ: 1.29 (s, 6 H), 1.93 (5 lines, 2 H, J = 6.5), 2.67 (t, 4 H, J = 6.9); 13C NMR (76 MHz, CDCl3) δ: 18.1, 22.3, 37.4, 61.8, 210.6.

DESIGNING A BETTER DRUG: The combination of chemical groups from three different fragments that bind weakly to an enzyme produce a potent new enzyme inhibitor (center) that binds in the nM range.COURTESY OF RODERICK HUBBARD
In search of better drugs and therapies, researchers are constantly looking for new ways to identify compounds that selectively block disease pathways. Industrial labs have relied on high-throughput screening to finger promising new molecules, but most academic labs lack the equipment and resources to scan many thousands, even millions, of compounds. For a long while this shut academic labs out of such searches, but a related technique, fragment-based drug discovery (also called fragment-based lead discovery), offers another way to develop small-molecule drugs and chemical probes for investigating biological processes. And this approach relies on instruments and expertise available at many academic institutions.
read at
A guide to fragment-based drug discovery
http://www.the-scientist.com/?articles.view/articleNo/35711/title/Piece-by-Piece/
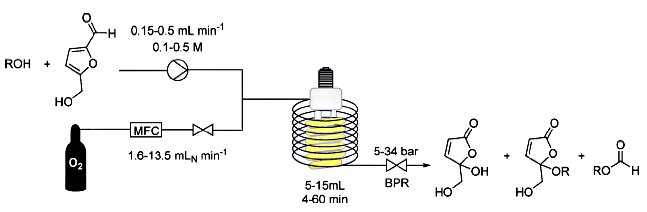
C. Oliver Kappe, University of Graz, Austria, and colleagues prepared for the first time the potential new platform molecule H2MF in pure form and converted it to the polyester precursor 5-hydroxy-4-keto-pentenoic acid (HKPA).
read at
http://www.chemistryviews.org/details/ezine/7176481/.html

C. Oliver Kappe
THE KAPPE LABORATORY
Institute of Chemistry, University of Graz, Austria
C. Oliver Kappe is Professor of Chemistry at the University of Graz, Austria. He received his diploma- (1989) and his doctoral (1992) degrees in organic chemistry from the University of Graz where he worked with Professor Gert Kollenz on cycloaddition and rearrangement reactions of acylketenes. After periods of postdoctoral research work on reactive intermediates and matrix isolation spectroscopy with Professor Curt Wentrup at the University of Queensland in Brisbane, Australia (1993-1994) and on synthetic methodology/alkaloid synthesis with Professor Albert Padwa at Emory University in Atlanta, USA (1994-1996), he moved back to the University of Graz in 1996 to start his independent academic career. He obtained his “Habilitation” in 1998 in organic chemistry and was appointed Associate Professor in 1999. Since 2011 he holds the position of Professor of “Technology of Organic Synthesis” (Organische Synthesetechnologie) at the Instittue of Chemistry at the University of Graz. He has spent time as visiting scientist/professor at e.g. the Scripps Research Institute (La Jolla, USA, Professor K. Barry Sharpless, 2003), the Toyko Institute of Technology (Toyko, Japan, Professor T. Takahashi, 2008), the University of Sassari (Sassari, Italy, 2008), the Sanford-Burnham Institute for Medical Research (Orlando, USA, 2010) and the Federal University of Rio de Janeiro (Ri de Janeiro, Brazil, 2013).
The co-author of ca. 350 publications, his main research interests have in the past focused on multicomponent reactions, combinatorial chemistry and the synthesis of biologically active heterocycles. More recently his research group has been involved with enabling and process intensification technologies, including microwave and continuous flow chemistry. For his innovative work in microwave chemistry he received the 2004 Prous Science Award from the European Federation for Medicinal Chemistry and the 2010 Houska Prize (100.000 €) in addition to a number of other awards.
C. Oliver Kappe is currently Editor-in-Chief of the Journal of Flow Chemistry (Akadémiai Kiadó) and a board member of the Flow Chemistry Society. In addition he has been an Editor of the Journal QSAR and Combinatorial Sciences (Wiley-VCH, 2003-2007) and has served/serves on the Editorial/Advisory Boards of the Journal of Combinatorial Chemistry (ACS), Molecular Diversity (Springer), ChemMedChem and ChemSusChem (Wiley-VCH), Journal of Heterocyclic Chemistry (Wiley-VCH) and a number of other journals.

SEE
http://oneorganichemistoneday.blogspot.in/2014/12/dr-c-oliver-kappe.html

CMI 977

(2S,5S)-1-[4-[5-(4-Fluorophenoxymethyl)tetrahydrofuran-2-yl]-3-butynyl]-1-hydroxyurea 175212-04-1 CMI-977 is a potent 5-lipoxygenase inhibitor that intervenes in the production of leukotrienes and is presently being developed for the treatment of chronic asthma. It is a single enantiomer with an all-trans (2S,5S) configuration. Of the four isomers of CMI-977, the S,Sisomer was found to have the best biological activity and was selected for further development. The enantiomerically pure product was synthesized on a 2-kg scale from (S)-(+)-hydroxymethyl-γ-butyrolactone.
CytoMed, Inc. announced y the initiation of Phase I clinical trials for CMI-977, its orally active therapeutic product for the treatment of asthma. CMI-977 inhibits the 5-lipoxygenase (5-LO) cellular inflammation pathway to block the generation of leukotrienes, which play a key role in triggering bronchial asthma. The Company also announced that it has received a U.S. patent covering a number of 5-LO inhibitor compounds, including CMI-977, and their use in treating inflammatory and other disorders.
"Asthma is a chronic, persistent inflammatory disease of the airways characterized by coughing and wheezing. These symptoms are induced by the release of inflammatory mediators, including leukotrienes, from inflammatory cells in the lining of the airways," said Colin Scott, Vice President, Clinical and Regulatory Affairs of CytoMed. "CMI-977 inhibits the production of all classes of leukotrienes by inhibiting the 5-LO pathway. Preclinical studies of CMI-977 have shown similar efficacy to steroid treatment in reducing inflammation, without any evidence of the significant toxicity that has been associated with long-term use of steroids."
"CytoMed's product development strategy focuses on leveraging its expertise in molecular biology, medicinal chemistry and pharmacology to develop a broad range of product candidates," commented Thomas R. Beck, M.D., Chairman and CEO of CytoMed. "Moving our second product into the clinic is a significant step towards the Company's goal of developing a portfolio of safe and efficacious anti-inflammatory compounds." The Company's lead product, CMI-392, is currently in Phase II studies in collaboration with Stiefel Laboratories as a topical treatment for inflammation-related skin disorders.
The Phase I trial of CMI-977, which involves 56 healthy human volunteers, is being conducted at a single site. The double blind, randomized, escalating single dose study is designed to assess CMI-977's safety and tolerability.
The Company plans to complete the study in mid-1998. Over 14.6 million Americans suffer from chronic asthma. The disease is characterized by a widespread narrowing of the airways due to a contraction (spasm) of smooth muscle and overproduction of mucous, which blocks the air passages. These changes are caused by the release of spasmogens and vasoactive substances, including leukotrienes. Current long-term therapies include corticosteroids, which function by non-selectively suppressing a variety of cellular pathways that initiate inflammation. Steroids, while often effective, are associated with significant adverse side effects. CMI- 977 is a leukotriene modulator, part of a new class of drugs designed to
provide patients with a viable alternative to steroids.
CytoMed, Inc. is a growing biopharmaceutical company committed to the discovery and development of novel proprietary products for the treatment of inflammatory disease. The Company has three products in clinical or preclinical stage of development: CMI-392 in Phase II studies for the treatment of inflammatory skin disorders in collaboration with Stiefel
Laboratories; CMI-977, an orally active product in Phase I clinical trials for the treatment of asthma; and CMI-CAB-2, in late-stage preclinical development for the treatment of acute pulmonary and cardiovascular inflammation. To date, the Company has been funded primarily by investments from institutional and venture investors including Schroder Ventures, Oracle Strategic Partners, Atlas Venture, CIP Capital, BioAsia Investors, WPG Farber, Gateway Ventures, HealthCare Ventures and New York Life Insurance.
http://pubs.acs.org/doi/abs/10.1021/op980209l
………………………… 
 A practical gram scale asymmetric synthesis of CMI-977 is described. A tandem double elimination of an α-chlorooxirane and concomitant intramolecular nucleophilic substitution was used as the key step. Jacobsen hydrolytic kinetic resolution and Sharpless asymmetric epoxidation protocols were applied for the execution of the synthesis of the key chiral building block.
A practical gram scale asymmetric synthesis of CMI-977 is described. A tandem double elimination of an α-chlorooxirane and concomitant intramolecular nucleophilic substitution was used as the key step. Jacobsen hydrolytic kinetic resolution and Sharpless asymmetric epoxidation protocols were applied for the execution of the synthesis of the key chiral building block.
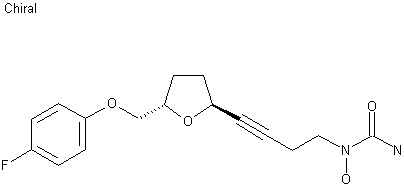
http://www.sciencedirect.com/science/article/pii/S0957416603001575 ……………………………….  The reaction of oxirane (I) with vinylmagnesium bromide in THF gives 1-(4-fluorophenoxy)-4-penten-2(S)-ol (II), which is treated with ethyl vinyl ether and mercuric trifluoroacetate to yield the vinyl ether (III). The cyclization of (III) by means of Grubb’s catalyst in refluxing benzene affords the dihydrofuran (IV), which is treated with benzenesulfinic acid in dichloromethane to give the sulfone (V). The reaction of (V) with the acetylenic tetrahydropyranyl ether (VI) by means of isopropylmagnesium bromide in THF yields the expected addition product (VII), which is treated with TsOH to eliminate the tetrahydropyranyl group and provide the alcohol (VIII). The condensation of (VIII) with N,O-bis (phenoxycarbonyl)hydroxylamine (IX) by means of PPh3 and DEAD in THF affords the protected carbamate derivative (X), which is finally treated with ammonia in methanol.
The reaction of oxirane (I) with vinylmagnesium bromide in THF gives 1-(4-fluorophenoxy)-4-penten-2(S)-ol (II), which is treated with ethyl vinyl ether and mercuric trifluoroacetate to yield the vinyl ether (III). The cyclization of (III) by means of Grubb’s catalyst in refluxing benzene affords the dihydrofuran (IV), which is treated with benzenesulfinic acid in dichloromethane to give the sulfone (V). The reaction of (V) with the acetylenic tetrahydropyranyl ether (VI) by means of isopropylmagnesium bromide in THF yields the expected addition product (VII), which is treated with TsOH to eliminate the tetrahydropyranyl group and provide the alcohol (VIII). The condensation of (VIII) with N,O-bis (phenoxycarbonyl)hydroxylamine (IX) by means of PPh3 and DEAD in THF affords the protected carbamate derivative (X), which is finally treated with ammonia in methanol. http://www.chemdrug.com/databases/8_0_sluqxnnnfcuabcvj.html
http://www.chemdrug.com/databases/8_0_sluqxnnnfcuabcvj.html
””””””””””””””””””””
![]()
http://www.scielo.br/scielo.php?pid=S0103-50532013000200003&script=sci_arttext Asthma is a chronic inflammatory disease of the respiratory system that results in the reduction or even the obstruction of air flow into the lungs.1 Over the last 40 years, there have been sharp increases in the global prevalence of asthma and the mortality due to this condition. In 2006, approximately 300 million people worldwide developed asthma, and there are approximately 180,000 deaths annually.2 In Brazil, asthma is the third most common cause of hospitalization in the Brazilian Unified Health System (SUS).3 The underdiagnosis and undertreatment of this disease have motivated the scientific community to search for new target-specific drugs to treat asthma and related respiratory diseases.4 The compound CMI-977 (LDP-977) (1) was discovered by Cyto-Med Inc., USA,5 and has been demonstrated to be a prominent candidate for the treatment of chronic asthma (Figure 1). This compound inhibits the 5-lipoxygenase pathway, thus blocking the production of leukotrienes.6 LDP-977 (1), containing a THF-2,5-trans-substituted ring with a (2S,5S) configuration, is orally active, and exhibits a good safety profile, a high degree of potency and excellent oral bioavailability relative to the three other stereoisomers.5

(2S,5S)-trans-5-[(4-Fluorophenoxy)methyl]-2-(4-N-hydroxyureidyl-1-butynyl)tetrahydrofuran, CMI-977 Over the years, several synthetic routes have been proposed for the stereoselective synthesis of the THF moiety present in CMI-977 (1) (Scheme 1).5,7,8  Intermediate 4 was prepared by Cyto-Med Inc., USA, using the first synthetic route developed,5 which involved a chiral pool approach for the creation of the C9 stereogenic center (Scheme 1). A nucleophilic attack involving an oxonium electrophile intermediate, obtained from 3, produced C6, but a disappointing low degree of selectivity was observed. In a similar oxonium strategy, Ley and co-workers7 employed an anomeric oxygen to promote the carbon rearrangement of an alkynyltributylstannane to access the THF unit, but their reaction also exhibited low selectivity (Scheme 1). Other similar strategies have led to similar results.8 Gurjar et al.9 reported a new stereoselective approach that installs the stereocenters at C6 and C9 in 6 using both Jacobsen hydrolytic kinetic resolution (HKR) and a Sharpless asymmetric epoxidation step (Scheme 1). The formation of a tandem propargyl alkoxide followed by intramolecular substitution resulted in the creation of the key tetrahydrofuran ring intermediate 7. Ley and co-workers10 also explored a similar tandem strategy providing the Retrosynthetic analysis of CMI-977 (LDP-977) (1) suitable intermediate 11, which in turn afforded the key fragment 7. These two new approaches were clearly Our disconnection approach began with a superior for the construction of the 2,5-anti THF unit as higher levels of diastereoselectivity were achieved. However, numerous steps are involved in these synthetic epoxide routes. In this paper, it is described our approach for the total synthesis of CMI-977 (LDP-977) (1). The biological importance of the target molecule and its structural features inspired us to devise a more concise and diastereoselective route to achieve the THF-2,5-trans ring of intermediate 7.
Intermediate 4 was prepared by Cyto-Med Inc., USA, using the first synthetic route developed,5 which involved a chiral pool approach for the creation of the C9 stereogenic center (Scheme 1). A nucleophilic attack involving an oxonium electrophile intermediate, obtained from 3, produced C6, but a disappointing low degree of selectivity was observed. In a similar oxonium strategy, Ley and co-workers7 employed an anomeric oxygen to promote the carbon rearrangement of an alkynyltributylstannane to access the THF unit, but their reaction also exhibited low selectivity (Scheme 1). Other similar strategies have led to similar results.8 Gurjar et al.9 reported a new stereoselective approach that installs the stereocenters at C6 and C9 in 6 using both Jacobsen hydrolytic kinetic resolution (HKR) and a Sharpless asymmetric epoxidation step (Scheme 1). The formation of a tandem propargyl alkoxide followed by intramolecular substitution resulted in the creation of the key tetrahydrofuran ring intermediate 7. Ley and co-workers10 also explored a similar tandem strategy providing the Retrosynthetic analysis of CMI-977 (LDP-977) (1) suitable intermediate 11, which in turn afforded the key fragment 7. These two new approaches were clearly Our disconnection approach began with a superior for the construction of the 2,5-anti THF unit as higher levels of diastereoselectivity were achieved. However, numerous steps are involved in these synthetic epoxide routes. In this paper, it is described our approach for the total synthesis of CMI-977 (LDP-977) (1). The biological importance of the target molecule and its structural features inspired us to devise a more concise and diastereoselective route to achieve the THF-2,5-trans ring of intermediate 7.  Results and Discussion Retrosynthetic analysis of CMI-977 (LDP-977) (1) Our disconnection approach began with a long-established strategy for the insertion of the N-hydroxy urea moiety by alkylation involving acetylene 7 and epoxide 13, followed by a Mitsunobu-like reaction involving alcohol 4 and hydroxycarbamate 12 (Scheme 2).9,10 The terminal acetylene 7 can be assembled via Seyferth-Gilbert homologation (using the Ohira-Bestmann protocol)11 involving the aldehyde prepared from alcohol 14. It was intended to create the trans-THF configuration in our key fragment 14 using a Mukaiyama oxidative cyclization protocol with homoallylic alcohol 15.12 The functional groups in fragment 15 could be installed starting from commercially available and inexpensive 4-fluorophenol 16, rac-epichlorohydrin 17 and allylbromomagnesium 18, in a strategy similar to that applied by Gurjar et al.9 Preparation of the key fragment 14 Our approach to the total synthesis of CMI-977 (LDP-977) (1) began with the reaction of p-fluorophenol 16 with rac-epichlorohydrin 17 in the presence of KOH, providing rac-5 in 97% yield (Scheme 3).13
Results and Discussion Retrosynthetic analysis of CMI-977 (LDP-977) (1) Our disconnection approach began with a long-established strategy for the insertion of the N-hydroxy urea moiety by alkylation involving acetylene 7 and epoxide 13, followed by a Mitsunobu-like reaction involving alcohol 4 and hydroxycarbamate 12 (Scheme 2).9,10 The terminal acetylene 7 can be assembled via Seyferth-Gilbert homologation (using the Ohira-Bestmann protocol)11 involving the aldehyde prepared from alcohol 14. It was intended to create the trans-THF configuration in our key fragment 14 using a Mukaiyama oxidative cyclization protocol with homoallylic alcohol 15.12 The functional groups in fragment 15 could be installed starting from commercially available and inexpensive 4-fluorophenol 16, rac-epichlorohydrin 17 and allylbromomagnesium 18, in a strategy similar to that applied by Gurjar et al.9 Preparation of the key fragment 14 Our approach to the total synthesis of CMI-977 (LDP-977) (1) began with the reaction of p-fluorophenol 16 with rac-epichlorohydrin 17 in the presence of KOH, providing rac-5 in 97% yield (Scheme 3).13  The epoxide rac-5was resolved by hydrolytic kinetic resolution under Jacobsen conditions,14 using the catalyst (R, R)-(salen)CoIII(OAc) (19, 0.5 mol%) and H2O (0.57 equiv) in tert-butyl methyl ether, providing (S)-5 in a 48% yield.9 The next step involved the epoxide ring-opening of (S)-5 with allylmagnesium bromide (18), providing homoallylic alcohol 15 in a quantitative yield (Scheme 4).
The epoxide rac-5was resolved by hydrolytic kinetic resolution under Jacobsen conditions,14 using the catalyst (R, R)-(salen)CoIII(OAc) (19, 0.5 mol%) and H2O (0.57 equiv) in tert-butyl methyl ether, providing (S)-5 in a 48% yield.9 The next step involved the epoxide ring-opening of (S)-5 with allylmagnesium bromide (18), providing homoallylic alcohol 15 in a quantitative yield (Scheme 4).  The subsequent oxidative cyclization of 15 according to the Mukaiyama protocol,12 mediated by the Co(modp)2 (20) (30 mol%) catalyst,15 provided trans-THF 14 as the only observed diastereoisomer in an 84% yield.8 This approach has proven to be a powerful strategy for accessing the 2,5-trans-THF unit in a highly diastereoselective fashion. Preparation of the key fragment 4 and conclusion of the synthesis The alcohol 14 was then oxidized to aldehyde 21 under Parikh-Doering conditions, followed by Seyferth-Gilbert homologation16 using the Ohira-Bestmann reagent 22,11 assembling the terminal acetylene 7 in a 75% yield over two steps (Scheme 5).
The subsequent oxidative cyclization of 15 according to the Mukaiyama protocol,12 mediated by the Co(modp)2 (20) (30 mol%) catalyst,15 provided trans-THF 14 as the only observed diastereoisomer in an 84% yield.8 This approach has proven to be a powerful strategy for accessing the 2,5-trans-THF unit in a highly diastereoselective fashion. Preparation of the key fragment 4 and conclusion of the synthesis The alcohol 14 was then oxidized to aldehyde 21 under Parikh-Doering conditions, followed by Seyferth-Gilbert homologation16 using the Ohira-Bestmann reagent 22,11 assembling the terminal acetylene 7 in a 75% yield over two steps (Scheme 5).  The 1H NMR and 13C NMR spectra and the optical rotation of trans-THF 7 matched the reported values for this compound.9 Next, the treatment of 7 with n-BuLi and ethylene oxide 13 led to alcohol 4 in a 70% yield. As shown in Scheme 5, the preparation of hydroxycarbamate 26 (53% yield), followed by its acetylation using acetyl chloride 27, provided 12 in a quantitative yield. A Mitsunobu-like reaction between alcohol 4 and N-hydroxycarbamate 12 provided 23 in a 93% yield. Finally, 23 was ammonolysed with NH3·MeOH, yielding CMI-977 as a white solid in a 38% yield. The spectral and physical data of the synthetic sample were in complete agreement with those reported in the literature.5,7-9
The 1H NMR and 13C NMR spectra and the optical rotation of trans-THF 7 matched the reported values for this compound.9 Next, the treatment of 7 with n-BuLi and ethylene oxide 13 led to alcohol 4 in a 70% yield. As shown in Scheme 5, the preparation of hydroxycarbamate 26 (53% yield), followed by its acetylation using acetyl chloride 27, provided 12 in a quantitative yield. A Mitsunobu-like reaction between alcohol 4 and N-hydroxycarbamate 12 provided 23 in a 93% yield. Finally, 23 was ammonolysed with NH3·MeOH, yielding CMI-977 as a white solid in a 38% yield. The spectral and physical data of the synthetic sample were in complete agreement with those reported in the literature.5,7-9
![]()
SPECTRAL DATA (2S,5S)-trans-5-[(4-Fluorophenoxy)methyl]-2-(4-N-hydroxyureidyl-1-butynyl)tetrahydrofuran, CMI-977 (1) To a round-bottomed flask, it was added 15 (85 mg, 0.19 mmol) at 0 ºC. Then, NH3 (2 mL, 14 mmol, 7 mol L-1in MeOH) was added, and the mixture was stirred at 0 ºC for 36 h. The reaction was concentrated under reduced pressure and purified by flash column chromatography using a mixture of CHCl3/MeOH (20:1) as the eluent, providing the compound CMI-977 (1) (24 mg, 0.074 mmol) as a colorless solid in a 38% yield; mp 106-107 ºC, 106-107 ºC;9
![]()
[α]D20 -40 (c 1.1, MeOH), [α]D -46.0 (c 1.1, MeOH);9
1H NMR (CDCl3, 250 MHz) δ 1.19 (s, 1H), 1.67-1.81 (m, 1H), 1.86-1.98 (m, 1H), 2.08-2.21 (m, 2H), 2.46 (t, 2H, J 6.5 Hz), 3.60 (t, 2H, J 6.8 Hz), 3.77-3.89 (m, 2H), 4.34-4.43 (m, 1H), 4.63-4.67 (m, 1H), 5.48 (s, 2H), 6.74-6.92 (m, 4H), 8.60 (br, 1H);
13C NMR (CDCl3, 150.9 MHz) δ 17.2 (CH2), 27.7 (CH2), 33.3 (CH2), 48.7 (CH2), 69.1 (CH), 70.7 (CH2), 76.9 (CH), 80.7 (C0), 82.9 (C0), 115.5 (CH), 115.7 (CH), 115.9 (CH), 154.8 (C0), 156.6 (C0), 158.2 (C0), 161.7 (C0);
IR (film) νmax/cm-1 3445, 3331, 3178, 2918, 2878, 1639, 1583, 1512, 1454, 1362, 1302, 1229, 1097, 1078, 1038, 937, 827, 762;
HRMS (ESI-TOF) m/z [M + H]+ for C16H20FN2O4 calcd. 323.1407, observed 323.1438.
![]()
References 1. Barnes P. J.; Br. J. Clin. Pharm. 1996,42, 3.
2. Braman, S. S.; Chest. 2006,130,4S. [ Links ]
3. Cabral, A. L. B.; Martins, M. A.; Carvalho, W. A. F.; Chinen,M.; Barbirotto, R. M.; Boueri, F. M. V.; Eur. Resp. J. 1998,12,35.
4. Jacobsen, J. R.; Choi, S. K.; Combs, J.; Fournier, E. J. L.; Klein, U.; Pfeiffer, J. W.; Thomas, G. R.; Yu, C.; Moran, E. J.; Bioorg. Med. Chem. Lett. 2012,22, 1213; [ Links ]
Millan, D. S.; Ballard, S. A.; Chunn, S.; Dybowski, J. A.; Fulton, C. K.; Glossop, P. A.; Guillabert, E.; Hewson, C. A.; Jones, R. M.; Lamb, D. J.; Napier, C. M.; Payne-Cook, T. A.; Renery, E. R.; Selby, M. D.; Tutt, M. F.; Yeadon, M.; Bioorg. Med. Chem. Lett.2011,21, 5826; [ Links ]
Sun, X. S.; Wasley, J. W. F.; Qiu, J; Blonder, J. P.; Stout, A. M.; Green, L. S.; Strong, S. A.; Colagiovanni, D. B.; Richards, J. P.; Mutka, S. C.; Chun, L.; Rosenthal, G. J.; ACS Med. Chem. Lett. 2011,2, 402; [ Links ]
Semko, C. M.; Chen, L.; Dressen, D. B.; Dreyer, M. L.; Dunn, W.; Farouz, F. S.; Freedman, S. B.; Holsztynska, E. J.; Jefferies, M.; Konradi, A. K.; Liao, A.; Lugar, J.; Mutter, L.; Pleiss, M. A.; Quinn, K. P.; Thompson, T.; Thorsett, E. D.; Vandevert, C.; Xu, Y.-Z.; Yednock, T. A.; Bioorg. Med. Chem. Lett .2011,21,1741. [ Links ]
5. Cai, X.; Hwang, S.; Killan, D.; Shen, T. Y.; US pat. 5,648,486 1997; [ Links ] Cai, X.; Grewal, G.; Hussion, S.; Fura, A.; Biftu, T.; US pat. 5,681,966 1997; [ Links ]
Cai, X.; Cheah, S.; Eckman, J.; Ellis, J.; Fisher, R.; Fura, A.; Grewal, G.; Hussion, S.; Ip, S.; Killian, D. B.; Garahan, L. L.; Lounsbury, H.; Qian, C.; Scannell, R. T.; Yaeger, D.; Wypij, D. M.; Yeh, C. G.; Young, M. A.; Yu, S.; Abs. Pap. Am. Chem. Soc.,1997,214,214-MEDI. [ Links ]
6. Cai, X.; Chorghade, M. S.; Fura, A.; Grewal, G. S.; Juaregui, K. A.; Lounsbury, H. A.; Scannell, R. T.; Yeh, C. G.; Young, M. A.; Yu, S.; Org. Process Res. Dev. 1999,3,73.
7. Dixon, D. J.; Ley, S. V.; Reynolds, D. J.; Chorghade, M. S.; Synth. Commun. 2000,30, 1955; [ Links ]Dixon, D. J.; Ley, S. V.; Reynolds, D. J.; Chorghade, M. S.; Indian J. Chem., Sect B 2001,40,1043.
8. Chorgade, M. S.; Gurjar, M. K.; Adikari, S. S.; Sadalapure, K.; Lalitha, S. V. S.; Murugaiah, A. M. S.; Radhakrishna, P.; Pure Appl. Chem. 1999,71, 1071; [ Links ] Gurjar, M. K.; Murali Krishna, L.; Sridhar Reddy, B.; Chorghade, M. S.; Synthesis 2000, 557; [ Links ] Chattopadhyay, A.; Vichare, P.; Dhotare, B.;Tetrahedron Lett. 2007,48,2871.
9. Gurjar, M. K.; Murugaiah, A. M. S.; Radhakrishna, P.; Ramana, C. V.; Chorghade, M. S.; Tetrahedron: Asymmetry 2003,14,1363.
10. Sharma, G. V. M.; Punna, S.; Prasad, T. R.; Krishna, P. R.; Chorghade, M. S.; Ley, S. V.; Tetrahedron: Asymmetry 2005,16,1113.
…………………………………………………
read
Pure Appl. Chem., Vol. 71, No. 6, pp. 1071-1074, 1999.
http://pac.iupac.org/publications/pac/pdf/1999/pdf/7106×1071.pdf
………………………………………………… 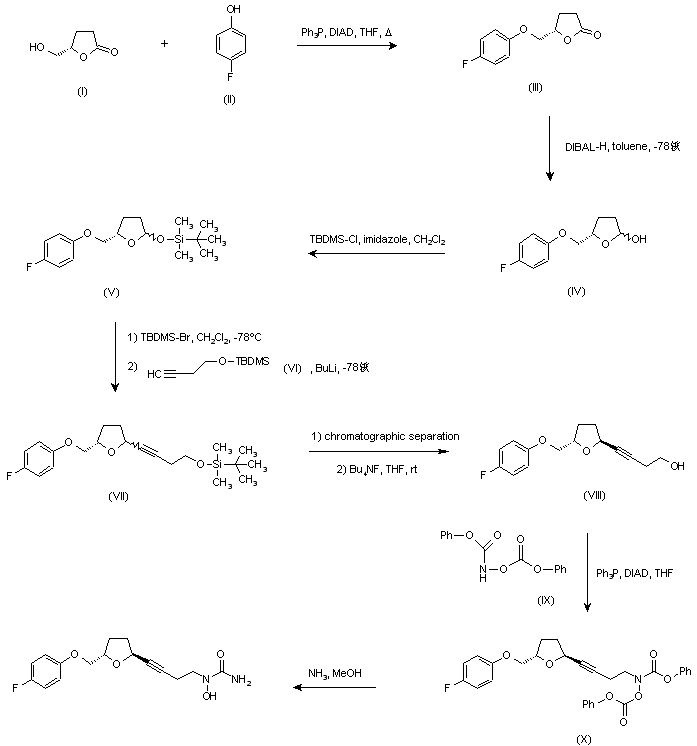 US 5703093; US 5792776; WO 9600212 Ether (III) was prepared by condensation of (S)-4-(hydroxymethyl)butyrolactone (I) and 4-fluorophenol (II) in the presence of diisopropylazodicarboxylate (DIAD) and triphenylphosphine under Mitsunobu conditions. Then, reduction of lactone (III) with DIBAL-H in toluene at -78 C gave lactol (IV), which was converted to silyl ether (V) by treatment with tert-butyldimethylsilyl chloride (TBDMS-Cl) and imidazole. Subsequent reaction of (V) with TBDMS-Br in CH2Cl2 at -78 C, followed by condensation with the lithium acetylide derived from acetylene (VI), yielded compound (VII) as a mixture of isomers. Chromatographic separation of the mixture provided the desired trans isomer, which was deprotected by treatment with tetra-n-butylammonium fluoride to give alcohol (VIII). This was then condensed with N,O-bis(phenoxycarbonyl)hydroxylamine (IX) in the presence of DIAD and Ph3P to furnish the hydroxamic acid derivative (X). Finally, concomitant deprotection of the O-phenoxycarbonyl group and substitution of the remaining phenoxy group for an amino group by treatment with methanolic ammonia in a pressure tube, provided the title compound.
US 5703093; US 5792776; WO 9600212 Ether (III) was prepared by condensation of (S)-4-(hydroxymethyl)butyrolactone (I) and 4-fluorophenol (II) in the presence of diisopropylazodicarboxylate (DIAD) and triphenylphosphine under Mitsunobu conditions. Then, reduction of lactone (III) with DIBAL-H in toluene at -78 C gave lactol (IV), which was converted to silyl ether (V) by treatment with tert-butyldimethylsilyl chloride (TBDMS-Cl) and imidazole. Subsequent reaction of (V) with TBDMS-Br in CH2Cl2 at -78 C, followed by condensation with the lithium acetylide derived from acetylene (VI), yielded compound (VII) as a mixture of isomers. Chromatographic separation of the mixture provided the desired trans isomer, which was deprotected by treatment with tetra-n-butylammonium fluoride to give alcohol (VIII). This was then condensed with N,O-bis(phenoxycarbonyl)hydroxylamine (IX) in the presence of DIAD and Ph3P to furnish the hydroxamic acid derivative (X). Finally, concomitant deprotection of the O-phenoxycarbonyl group and substitution of the remaining phenoxy group for an amino group by treatment with methanolic ammonia in a pressure tube, provided the title compound. http://www.chemdrug.com/databases/8_0_sluqxnnnfcuabcvj.html …………………………………………………. PAPER
http://www.chemdrug.com/databases/8_0_sluqxnnnfcuabcvj.html …………………………………………………. PAPER
| Title: | A short and efficient stereoselective synthesis of the potent 5-lipoxygenase inhibitor, CMI-977† |
| Authors: | Dixon, Darren J Ley, Steven V Reynolds, Dominic J Chorghade, Mukund S |
| Issue Date: | Nov-2001 |
| Publisher: | NISCAIR-CSIR, India |
| Abstract: | A short and efficient synthesis of the potent 5-lipoxygenase inhibitor CMI-977 has been accomplished, utilising an oxygen to carbon rearrangement of an anomerically linked alkynyl stannane tetrahydrofuranyl ether derivative as the key step. |
| Page(s): | 1043-1053 |
| CC License: |  CC Attribution-Noncommercial-No Derivative Works 2.5 India CC Attribution-Noncommercial-No Derivative Works 2.5 India |
| Source: | IJC-B Vol.40B(11) [November 2001] |
Files in This Item:
|
http://nopr.niscair.res.in/bitstream/123456789/22437/1/IJCB%2040B%2811%29%201043-1053.pdf ……………………………………………….

http://www.google.com.ar/patents/US20080081835 Specific inhibitors of 5-LO that may be mentioned include the following.
…………………………………..
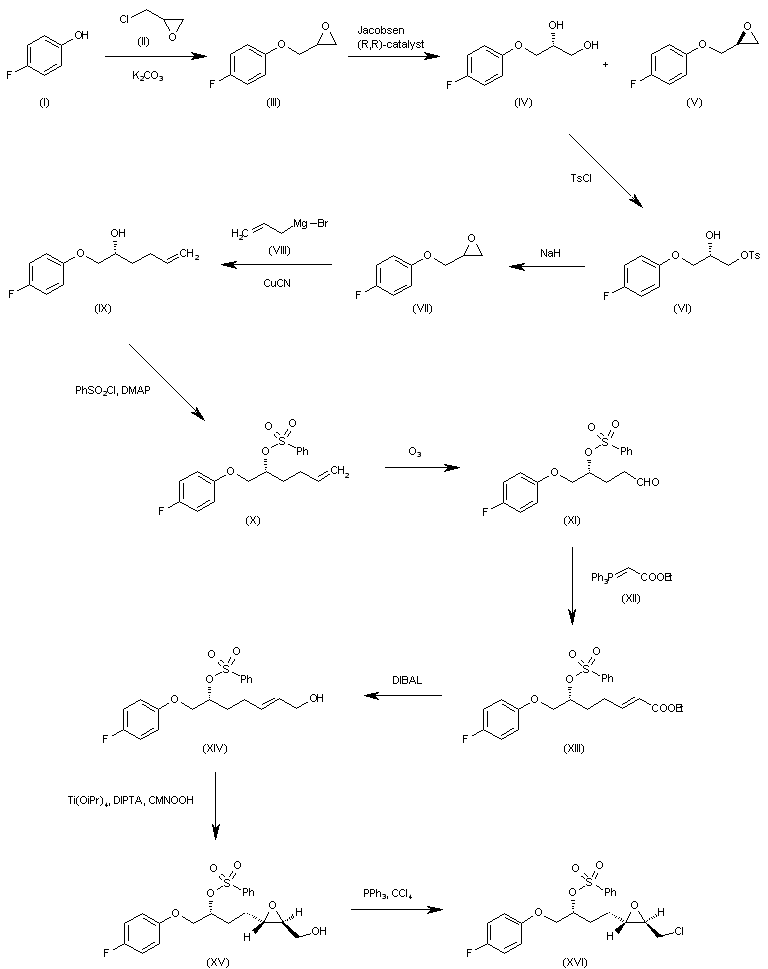 WO 0001381 The reaction of 4-fluorophenol (I) with epichlorohydrin (II) by means of K2CO3 in refluxing acetone gives 2-(4-fluorophenoxymethyl)oxirane (III), which is submitted to an enantioselective ring opening with the Jacobsen (R,R)-catalyst yielding a mixture of the (R)-diol (IV) and unaltered epoxide (V), easily separated by column chromatography. The reaction of (IV) with tosyl chloride and pyridine in dichloromethane affords the primary monotosylate (VI), which is converted into the chiral epoxide (VII) by reaction with NaH in THF/DMF. The reaction of (VII) with allylmagnesium bromide (VIII) in ethyl ether gives the 2-hexenol derivative (IX), which is treated with benzenesulfonyl chloride and DMAP yielding the sulfonate (X). The ozonolysis of (X) with ozone in dichloromethane affords the aldehyde (XI), which is condensed with ethoxycarbonylmethylene(triphenyl)phosphorane (XII) yielding the 2-heptenoic ester (XIII). The reduction of (XIII) with diisobutylaluminum hydride (DIBAL) in toluene/dichloromethane provides the 2-hepten-1-ol (XIV), which is epoxidized with cumene hydroperoxide in the presence of diisopropyl (+)-tartrate and Ti(Oi-Pr)4 in dichloromethane to give the chiral epoxyalcohol (XV). The reaction of (XV) with triphenylphosphine/CCl4 in chloroform affords the corresponding chloride (XVI). …………………………………….
WO 0001381 The reaction of 4-fluorophenol (I) with epichlorohydrin (II) by means of K2CO3 in refluxing acetone gives 2-(4-fluorophenoxymethyl)oxirane (III), which is submitted to an enantioselective ring opening with the Jacobsen (R,R)-catalyst yielding a mixture of the (R)-diol (IV) and unaltered epoxide (V), easily separated by column chromatography. The reaction of (IV) with tosyl chloride and pyridine in dichloromethane affords the primary monotosylate (VI), which is converted into the chiral epoxide (VII) by reaction with NaH in THF/DMF. The reaction of (VII) with allylmagnesium bromide (VIII) in ethyl ether gives the 2-hexenol derivative (IX), which is treated with benzenesulfonyl chloride and DMAP yielding the sulfonate (X). The ozonolysis of (X) with ozone in dichloromethane affords the aldehyde (XI), which is condensed with ethoxycarbonylmethylene(triphenyl)phosphorane (XII) yielding the 2-heptenoic ester (XIII). The reduction of (XIII) with diisobutylaluminum hydride (DIBAL) in toluene/dichloromethane provides the 2-hepten-1-ol (XIV), which is epoxidized with cumene hydroperoxide in the presence of diisopropyl (+)-tartrate and Ti(Oi-Pr)4 in dichloromethane to give the chiral epoxyalcohol (XV). The reaction of (XV) with triphenylphosphine/CCl4 in chloroform affords the corresponding chloride (XVI). …………………………………….
![]()
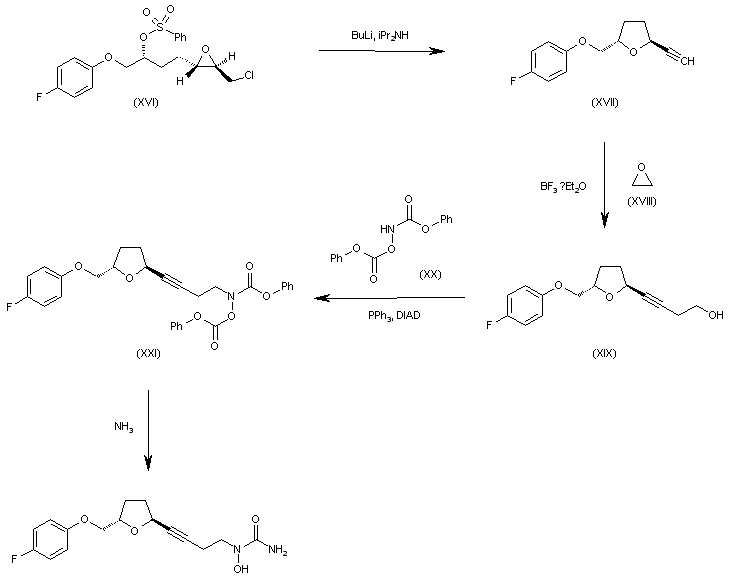 WO 0001381 Intermediate (XVI) is treated with BuLi and diisopropylamine in THF giving the chiral acetylenic tetrahydrofuran (XVII). The addition of ethylene oxide (XVIII) to the terminal acetylene of (XVII) by means of BF3/Et2O in THF gives the 3-butyl-1-ol derivative (XIX), which is condensed with N,O-bis(phenoxy- carbonyl)hydroxylamine (XX) by means of PPh3 and diisopropylazodicarboxylate (DIAD) in THF yielding the final intermediate (XXI). Finally, this compound is treated with ammonia in methanol to obtain the target urea derivative.
WO 0001381 Intermediate (XVI) is treated with BuLi and diisopropylamine in THF giving the chiral acetylenic tetrahydrofuran (XVII). The addition of ethylene oxide (XVIII) to the terminal acetylene of (XVII) by means of BF3/Et2O in THF gives the 3-butyl-1-ol derivative (XIX), which is condensed with N,O-bis(phenoxy- carbonyl)hydroxylamine (XX) by means of PPh3 and diisopropylazodicarboxylate (DIAD) in THF yielding the final intermediate (XXI). Finally, this compound is treated with ammonia in methanol to obtain the target urea derivative.
…………………………….
poster
http://www.prp.rei.unicamp.br/pibic/congressos/xxcongresso/paineis/092085.pdf
SÍNTESE TOTAL DO CMI-977 (LDP-977), UM PODEROSO AGENTE ANTIASMÁTICO
Lui Strambi Farina (IC), Marco Antonio Barbosa Ferreira (PG) e Luiz Carlos Dias (PQ)*
INSTITUTO DE QUÍMICA, UNIVERSIDADE ESTADUAL DE CAMPINAS, C.P. 6154, 13084-971, CAMPINAS, SP, BRASIL
*ldias@iqm.unicamp.br
Agência Financiadora: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ).
Palavras-Chave: Síntese orgânica, Tetrahidrofuranos, CMI-977 (LDP-977)
……………………………
https://www.thieme-connect.de/DOI/DOI?10.1055/s-0033-1338934
A total synthesis of (+)-muricatacin and a formal synthesis of CMI-977 have been achieved using commercially available l-malic acid based on our furan approach to oxacyclic systems, the proven scope of which is thus broadened.
Temperature control plays an important role in industrial processes, pilot plants, and chemical and pharmaceutical laboratories. When controlling reactors, both exothermic and endothermic reactions must be offset with high speed and reliability. Therefore, different conditions and effects must be taken into account when specifying an optimum and highly dynamic temperature control system.
.gif)
Most temperature control systems are used with chemical reactors made of either steel or glass. The former is more rugged and long-lasting, while the latter enables chemists to observe processes inside the reactor.
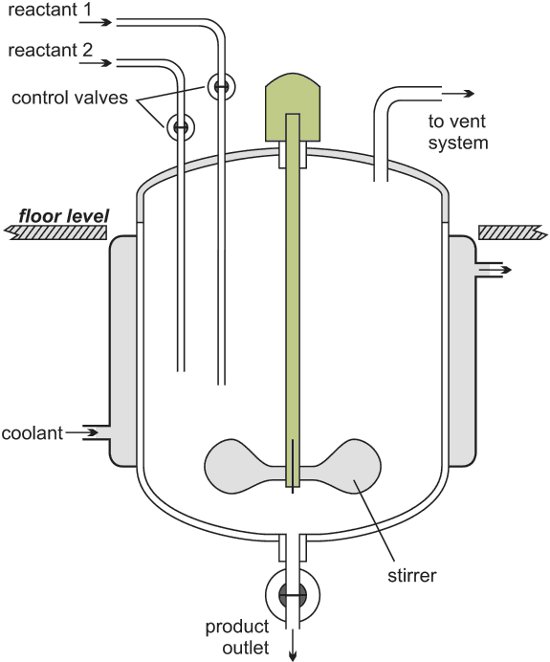
However, in the case of glass reactors, extensive precautions have to be followed for safe usage. Reactors usually include an inner vessel to hold the samples, which need temperature control. This inner vessel is enclosed by a jacket containing heat-transfer liquid. This reactor jacket is linked to the temperature control system.
In order to control the reactor’s temperature, the temperature control system pumps the heat-transfer liquid through the reactor’s jacket. Rapid temperature change inside the reactor is balanced by instant cool-down or heat-up, and the liquid is either cooled or heated inside the temperature control system. Figure 1 shows a schematic of a simple temperature control system.
.jpg)
Figure 1. Functional view of reactor temperature control
Both materials and reactor design can affect the temperature control of highly dynamic reactor systems. However, the heat transferred by a glass-walled vessel will be different than that transferred by a steel-walled vessel. In addition, both wall thickness and surface area can also affect accuracy. Therefore, proper mixing of the initial materials inside the reactor is important to obtain good uniformity, which in turn will guarantee optimal heat exchange.
For each type of reactor, maximum pressure values have been provided as per the specifications established by reactor manufacturers and in the Pressure Equipment Directive 97/23/EG. Regardless of any temperature control application, these limit values may not be surpassed during operation under any situations. Prior to starting a temperature control application, the applicable limits must be programmed within the temperature control unit.

Another important criterion in reactors is the maximum permissible temperature difference, which is referred to as Delta-T limit. It defines the highest difference between the temperature of the contents of the reactor and the actual thermal fluid temperature.
When compared to steel reactors, glass reactors are more susceptible to thermal stress. For that matter, any temperature control system should enable users to program reactor-specific values for the Delta-T limit per time unit. Within the temperature control equipment itself, three components considerably affect the stability of the process and these include heat exchanger, pump, and control electronics.
It is important to ensure that a temperature control system has sufficient heating and cooling capacity, as this can significantly affect the speed to reach the preferred temperatures. In order to determine the preferred heating and cooling capacities, users must consider the essential differences in temperature, the volume of the samples, the preferred heat-up and cool-down times, and the specific heat capacity of the temperature control medium.
Highly dynamic temperature control solutions are commercially available in the market with water or air cooling. Air-cooled systems do not utilize water and may be deployed where there is sufficient air flow.
The heat thus removed from the reactor is eventually transferred to ambient air. Water-cooled systems need to be joined to a cooling water supply, but they operate more quietly and do not add surplus heat in small labs. These units could be completely enclosed by the application, if required.
The integrated pump of the temperature control unit equipment must be sufficiently strong to obtain the preferred flow rates at stable pressure. To ensure that pressure limit values mentioned above are not exceeded, the pump should provide the preferred pressure quickly and with maximum control.
Operating conditions and pressure specifications of the reactor must always be taken into account, and regulation of pump capacity must be done by presetting a limit value. Sophisticated temperature control solutions include pumps that balance the variations of the viscosity of the heat transfer liquid to make sure that energy efficiency is maintained continuously.
This is because viscosity influences flow and hence the heat transfer. An additional advantage provided by magnetically coupled pumps is that they guarantee a hydraulically-sealed thermal circuit. Also, self-lubricated pumps are beneficial as they require only minimum maintenance.
The closed loop circuit prevents contact between the ambient air and the heat transfer liquid. This not only prevents permeation of oxidation and moisture, bit also prevents oil vapors from entering into the work environment.
Additionally, an internal expansion vessel must permanently absorb temperature-induced volume variations inside the heat exchanger. Individual cooling of the expansion vessel will help in ensuring that the temperature control unit does not overheat and ultimately ensures operator safety.
A temperature control equipment should operate consistently even at high ambient temperatures. In majority of cases, the real work environment will diverge from the ideal temperature of 20°C. During hot summer months, temperature control solutions are exposed to adverse conditions. In laboratories, ambient temperatures are usually higher because of energy saving measures. These instances demonstrate the benefits of temperature control solutions that work consistently at temperatures as high as 35°C.

Temperature control equipment includes advanced control electronics that monitor and control the process inside the reactor and also the internal processes of the system. When a control variable changes, the system is capable of readjusting the variable to the setpoint sans overshooting.
Accurate control electronics are needed to maintain the stability of a temperature control application. One option to assess control electronics is to look at the effort needed to set parameters. In a temperature control unit, users can enter a setpoint. Control electronics must be self-optimizing throughout the temperature control process to ensure optimum results.

To sum up, the process safety and stability during reactor temperature control relies on the effectiveness of heat transfer, the type of reactor, and the efficiency of the components within the temperature control unit. Therefore, different conditions and effects must be considered when specifying a highly dynamic temperature control system.


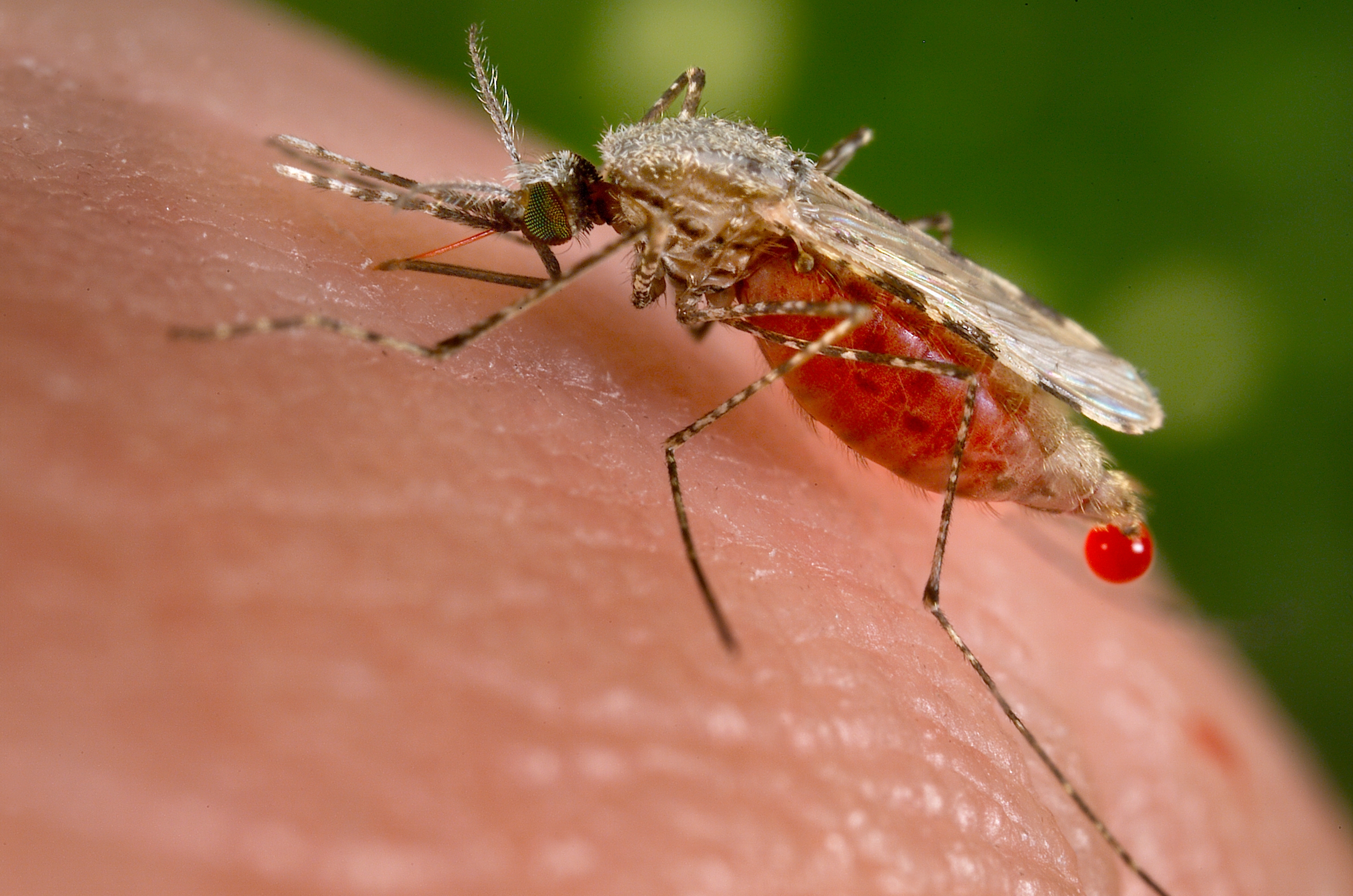
Malaria, a devastating infectious disease caused by Plasmodium spp., leads to roughly 655,000 deaths per year, mostly of African children. To compound the problem, drug resistance has emerged to all classical antimalarials and may be emerging for artemisinin-based combination therapies. To address the need for new antimalarials with novel mechanisms, several groups carried out phenotypic screening campaigns to identify compounds inhibiting growth of the blood stages of Plasmodium falciparum. In this review, we describe the characterization of these compounds, explore currently ongoing strategies to develop lead molecules, and endorse the concept of a “malaria box” of publicly accessible active compounds.

Malaria is a mosquito-borne disease that kills roughly 655,000 people every year, mostly young children in Africa. Malaria affects roughly 215 million patients annually (World Health Organization, 2011), and approximately one third of the world’s population is at risk for contracting the disease. The World Health Organization has announced a new campaign for global malaria eradication (Wells et al., 2009).
Mosquito-borne diseases are usually controlled by a combination of vector control, vaccines, and chemotherapy. In the case of malaria, economical vector control strategies, including insecticide-impregnated bed nets and localized spraying, have been deployed with success (Okumu and Moore, 2011). Additionally, progress is being made toward effective vaccines with the RTSS vaccine from GlaxoSmithKline (GSK), giving some protection (Schwenk and Richie, 2011). Nonetheless, chemotherapy remains the dominant component of malaria control. Unfortunately, clinical resistance has emerged for most available drugs (Petersen et al., 2011), and there are recent indications of the emergence of resistance to the artemisinin components of artemisinin-based combination therapies, which are a cornerstone of current antimalarial treatment strategies (Dondorp et al., 2009, Mok et al., 2011, Saralamba et al., 2011 and Veiga et al., 2011).

Therefore, new antimalarials are urgently needed. The focus of the discovery process is on new medicines that are structurally distinct from existing drugs, act by novel mechanisms, and avoid being acted upon by drug transporters overexpressed or overactive in multi-drug-resistant malaria. In the late 2000s, three groups, one in academia (St. Jude Children’s Research Hospital) (Guiguemde et al., 2010) and two in industry (GSK [Gamo et al., 2010] and Novartis [Plouffe et al., 2008]), identified novel leads using screening campaigns measuring the growth inhibitory potential of compounds acting on Plasmodium falciparum co-cultured during its asexual stages in human erythrocytes.
In this review, we discuss the driving force for conducting these screens; the results, including similarities and differences between the compounds identified; and the need for further innovation and work in understanding the underlying cellular and physiologic mechanisms by which the new classes of antimalarials work.

SEE………...http://cdn.thehoopla.com/images/68/0/raw/Gobal.Malaria.Pipeline.2013.pdf
Volume 19, Issue 1, 27 January 2012, Pages 116–129
High-Priority Series from Each Group
| Molecule | Series Example | Institution | Development Stage |
|---|---|---|---|
 |
Dihydropyridine SJ000025081 | SJCRH | Lead optimization |
 |
Diaminonaphthoqinone SJ000030570 | SJCRH | Lead optimization |
 |
Dihydroisoquinoline SJ000101247 | SJCRH | Preclinical |
 |
Carboxamide GSK2611622A | GSK | Lead optimization |
 |
Indoline TCMDC-139046 | GSK | Lead optimization |
 |
Alkylpyrazole TCMDC-134142 | GSK | Lead optimization |
 |
Thienopyrazole TCMDC-123580 | GSK | Lead optimization |
 |
Aminopiperidine TCMDC-124833 | GSK | Lead optimization |
 |
Spiroindolone NITD609 | Novartis | Phase I |
 |
Imidazolo piperazine | Novartis | Preclinical |
 |
Benzamide | Novartis | Lead optimization |
 |
Pyrimidine-4,6-diamine | Novartis | Lead optimization |
Anthony et al. Malaria Journal 2012 11:316 doi:10.1186/1475-2875-11-316
http://www.akademiai.com/content/r1t41145nn051252/?p=27bf59e482ec4093985c1e9ec3df3aba&pi=1
| Flow Chemistry | |
| Issue | Volume 1, Number 2/December 2011 |
| Pages | 56-61 |
| DOI | 10.1556/jfchem.2011.00013 |
1Structural Chemistry, Advanced Technology, Global Research and Development, Abbott Laboratories 100 Abbott Park Road 60064 Abbott Park, IL USA
2Medicinal Chemistry Technologies, Advanced Technology, Global Research and Development, Abbott Laboratories 100 Abbott Park Road 60064 Abbott Park, IL USA
3Automation Engineering, Advanced Technology, Global Research and Development, Abbott Laboratories 100 Abbott Park Road 60064 Abbott Park, IL USA
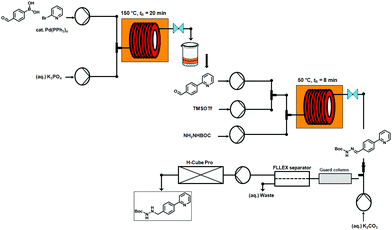
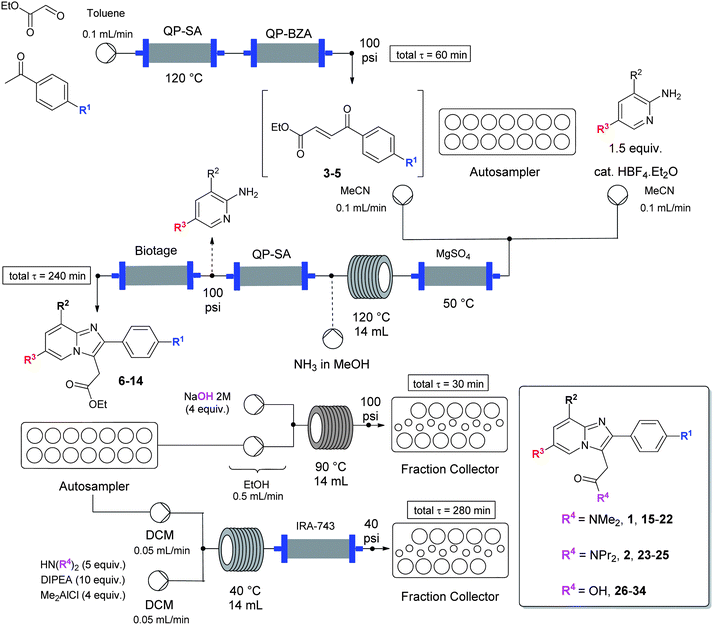
We report herein a high-throughput integrated ynthesis–purification platform termed SWIFT (synthesis with integrated-flow technology) and processes that accelerate the rate at which validated small-molecule organic compounds are generated. A segmented-flow synthesizer was integrated to a preparative HPLC-MS, where each reaction product was purified immediately upon reaction completion. Further, automated structure-validation processes accelerate the rate at which drug discovery candidates are available for biological screening.

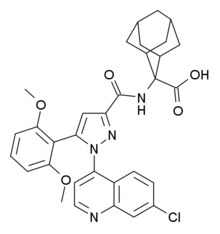
2-[[1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxyphenyl)pyrazole-3-carbonyl]amino]adamantane-2-carboxylic acid
Meclinertant (SR-48692) is a drug which acts as a selective, non-peptide antagonist at the neurotensin receptor NTS1, and was the first non-peptide antagonist developed for this receptor.[1][2] It is used in scientific research to explore the interaction between neurotensin and other neurotransmitters in the brain,[3][4][5][6][7][8] and produces anxiolytic, anti-addictive and memory-impairing effects in animal studies.[9][10][11][12]
PatentSubmittedGranted1-(7-chloroquinolin-4-yl)pyrazole-3-carboxamide N-oxide derivatives, method of preparing them, and their pharmaceutical compositions [US5561234]1996-10-01
Substituted 1-naphthyl-3-pyrazolecarboxamides which are active on neurotensin [US5585497]1996-12-17
3-amidopyrazole derivatives, process for preparing these and pharmaceutical composites containing them [US5420141]1995-05-30
Substituted 1-naphthyl-3-pyrazolecarboxamides which are active on neurotensin, their preparation and pharmaceutical compositions containing them [US5523455]1996-06-04
3-amidopyrazole derivatives, process for preparing these and pharmaceutical compositions containing them [US5607958]1997-03-04
3-amidopyrazole derivatives, process for preparing these and pharmaceutical compositions containing them [US5616592]1997-04-01
3-amidopyrazole derivatives, process for preparing these and pharmaceutical compositions containing them [US5635526]1997-06-03
Substituted 1-phenyl-3-pyrazolecarboxamides active on neurotensin receptors, their preparation and pharmaceutical compositions containing them [US5965579]1999-10-12
| Systematic (IUPAC) name | |
|---|---|
| 2-([1-(7-Chloro-4-quinolinyl)-5-(2,6-dimethoxyphenyl)-1H-pyrazole-3-carbonyl]amino)admantane-2-carboxylic acid | |
| Clinical data | |
| Legal status |
?
|
| Identifiers | |
| CAS number | 146362-70-1  |
| ATC code | ? |
| PubChem | CID 119192 |
| IUPHAR ligand | 1582 |
| UNII | 5JBP4SI96H  |
| Chemical data | |
| Formula | C32H31ClN4O5 |
| Mol. mass | 587.064 |

Dr. Claudio Battilocchio, Benjamin J. Deadman, Dr. Nikzad Nikbin, Dr. Matthew O. Kitching, Prof. Ian R. Baxendale and Prof. Steven V. Ley
Article first published online: 16 APR 2013 | DOI: 10.1002/chem.201300696
Flow and pharmaceuticals? An investigation into whether machine-assisted technologies can be of true help in the multistep synthesis of a potent neurotensin receptor-1 probe, Meclinertant (SR48692; see structure), is reported.
Meclinertant (SR 48692)
We developed an improved synthesis of the neurotensin antagonist biological probe SR 48692. The preparation includes an number of chemical conversions and strategies involving the use of flow chemistry platforms which helped overcome some of the limiting synthetic transformations in the original chemical route .
Meclinertant (SR 48692): The synthesis of neurotensin antagonist SR 48692 for prostate cancer research I.R. Baxendale, S. Cheung, M.O. Kitching, S.V. Ley, J.W. Shearman Bio. Org. Med. Chem. 2013, 21, 4378-4387.
A synthesis of the neurotensin 1 receptor probe Merclinertant (SR48692) has been reported using a range of continuous flow through synthesis, in-line reaction monioring and purification techniques. This strategy has been contrasted with a more conventional batch synthesis approach.
Notably the safe use of phosgene gas (generated in situ), the superheating of solvents to accelerate reaction rates, the processing of a reagent suspension under continuous flow-through conditions and the application of semi-permeable membrane technology to facilitate work-up and purification were all techniques that could be beneficially applied in the synthetic scheme.
Abstract:
Meclinertant, Reminertant, SR-48692
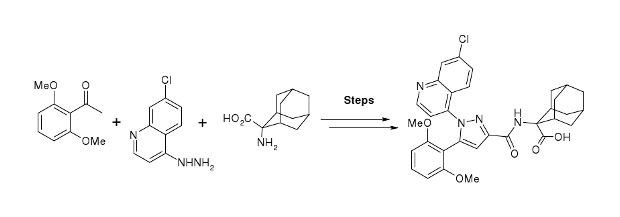
The condensation of 2′,6′-dimethoxyacetophenone (I) with diethyl oxalate (II) by means of sodium methoxide in refluxing methanol gives the dioxobutyrate (III), which is cyclized with 7-chloroquinoline-4-hydrazine (IV) in refluxing acetic acid yielding the pyrazole derivative (V). The hydrolysis of the ester group of (V) with KOH in refluxing methanol/water affords the corresponding carboxylic acid (VI), which is finally treated with SOCl2 in refluxing toluene and condensed with 2-aminoadamantane-2-carboxylic acid.
EP 0477049; FR 2665898; JP 1992244065; US 5420141; US 5607958; US 5616592; US 5635526; US 5744491; US 5744493
…………………………….

A report (Org Process Res Dev 2014, ASAP article) out of Jamison’s group at MIT, provides a 3-step synthesis of Rufinamide in 92% overall yield. The process illustrates a continuous and convergent method, moving away from the isolation of a key organic azide intermediates and a Cu coiled-tube reactor for the cycloaddition reaction to the corresponding desired triazole.
http://pubs.acs.org/doi/abs/10.1021/op500166n
Small molecules bearing 1,2,3-triazole functionalities are important intermediates and pharmaceuticals. Common methods to access the triazole moiety generally require the generation and isolation of organic azide intermediates. Continuous flow synthesis provides the opportunity to synthesize and consume the energetic organoazides, without accumulation thereof. In this report, we described a continuous synthesis of the antiseizure medication rufinamide. This route is convergent and features copper tubing reactor-catalyzed cycloaddition reaction. Each of the three chemical steps enjoys significant benefits and has several advantages by being conducted in flow. The total average residence time of the synthesis is approximately 11 min, and rufinamide is obtained in 92% overall yield.

Give it a flow: A continuous-flow process for the synthesis of a 1,2,3-triazole precursor of Rufinamide has been developed. The protocol involves a solvent- and catalyst-free operation and utilizes reaction temperatures above the melting point of the target product to prevent microreactor clogging, resulting in a decrease of the operating time from hours to minutes.
Article first published online: 23 SEP 2013
DOI: 10.1002/cssc.201300684
http://onlinelibrary.wiley.com/doi/10.1002/cssc.201300684/abstract


1…………………
Gleevec, developed by Novartis, is a tyrosine kinase inhibitor used for the treatment of chronic myeloid leukaemia and gastrointestinal stromal tumours. The drug molecule represents a particularly challenging target for flow chemistry because of the low solubility of many of the reaction components required for its synthesis. The team devised a new synthesis route that prevents the equipment blockages from product precipitation and avoids many of the labour and time intensive practices of traditional batch-based preparation.
2…………………..

Malaria is a serious global health issue. Artemisinin combination treatments are the first-line drugs, but supplies are limited because artemisinin is obtained solely by extraction from Artemisia annua. A continuous-flow process that converts dihydroartemisinic acid into artemisinin (see scheme) was shown to be an inexpensive and scalable process that can ensure a steady, affordable supply of artemisinin.
Article first published online: 16 JAN 2012
DOI: 10.1002/anie.201107446………….http://onlinelibrary.wiley.com/doi/10.1002/anie.201107446/abstract

IMAGE………..http://phys.org/news/2013-08-chemists-fresh-approach-alloy-nanomaterials.html
3……………….
http://www.beilstein-journals.org/bjoc/single/articleFullText.htm?publicId=1860-5397-9-265
IMAGE……..http://www.chemistryviews.org/details/ezine/1058453/Women_in_ChemistryA_European_Journal.html
4…………………….
_630m.jpg)
http://www.rsc.org/chemistryworld/2014/09/antimalarial-flow-synthesis-commercialisation-artemisinin

5…………………………….

http://pipeline.corante.com/archives/2014/04/

6…………………………

http://pubs.rsc.org/en/content/articlelanding/2013/ob/c2ob27003j#!divAbstract

7…………………..

http://onlinelibrary.wiley.com/doi/10.1002/anie.201305429/abstract

http://www.rsc.org/chemistryworld/2012/04/iron-lady
8………………………..

http://www-medchem.ch.cam.ac.uk/hot_topics.php

http://www.ollusa.edu/s/1190/ollu.aspx?pgid=2674
9…………………………

http://www.mdpi.com/1420-3049/19/7/9736

http://www.ed.ac.uk/alumni/services/news/news/femalechemists
10…………………
http://www3.imperial.ac.uk/newsandeventspggrp/imperialcollege/newssummary/news_26-9-2013-11-6-53
11……………………
http://pubs.rsc.org/en/content/articlehtml/2013/ob/c3ob41464g
12………………………..
http://pubs.rsc.org/en/content/articlelanding/2012/sc/c2sc21850j#!divAbstract

IMAGE……..http://evnewsreport.com/tag/battery/
13……………….

http://www.leygroup.ch.cam.ac.uk/research/continuous-flow-methodology/heterocycles-flow

14………………..

http://www.sfu.ca/chemistry/groups/britton/publications.html

IMAGE……….http://www.greentechnolog.com/green_chemistry/



Cyanohydrins are synthetically versatile chiral building blocks in organic synthesis. They can be conveniently synthesized in enantiomerically pure form via chemoenzymatic hydrogen cyanide addition onto the corresponding aldehyde using hydroxynitrile lyase.
Recently, we reported that such transformations can be efficiently carried out in a continuous flow manner using microreactors. Since racemization of enantiopure cyanohydrins occurs readily under slightly basic conditions, they should be protected before the follow-up reactions, preferably under acidic conditions.
In this contribution, we demonstrate that the methoxyisopropyl protection of mandelonitrile can be conveniently optimized in an automated microscale continuous flow system and subsequently scaled up under the same conditions by applying a larger flow reactor.

http://www.akademiai.com/content/9488206462627n38/?p=6ed413d7b9fb47fe9fe7e1262c37694f&pi=2
| Journal of Flow Chemistry | |
| Publisher | Akadémiai Kiadó |
| ISSN | 2062-249X (Print) 2063-0212 (Online) |
| Subject | Flow Chemistry |
| Issue | Volume 2, Number 4/December 2012 |
| Pages | 124-128 |
| DOI | 10.1556/JFC-D-12-00008 |

1Institute for Molecules and Materials Radboud University Nijmegen Heyendaalseweg 135 6525 AJ Nijmegen the Netherlands
 Floris P.J.T. Rutjes
Floris P.J.T. Rutjes


The IMM-office is located on the 3rd floor of the Huygens building, which is at walking distance (about 5 min.) from the railway station Nijmegen Heyendaal.

![]()
“Oleocanthal” is specifically deacetoxydialdehydic ligstroside aglycone, which exists as a single isomer (enantiomer). The (-)-enantiomer is the natural product and has the following chemical formula:
http://www.google.com/patents/EP2583676A1?cl=en
The Trustees of The University of Pennsylvania,
Monell Chemical Senses Center,
Russell S. J. Keast, Qiang Han, Amos B. Smith Iii, Gary K. Beauchamp, Paul A. S. Breslin, Jianming Lin,
employing a series of 1 and 2D NMR experiments (Andrewes, P. et al. (2003) J. Agric. Food Chem. 57:1415-1420), in conjunction with comparison to literature data (Montedoro, G. et al. (1993) J. Agric. Food Chem. 41:2228-2234). The absolute stereochemistry remained undetermined. That 1 was responsible for the strong pungent (burning) sensation at the back of the throat was based on an extensive series of HPLC fraction analysis, omission analysis and correlation, and hydrolysis studies, in conjunction with human sensory studies. Andrewes et al., however, acknowledged that “a coelution compound causing the burning sensation” could not be eliminated without completing a synthesis of 1, which they stated to be “extremely challenging.”
EXAMPLES
Example 1: Isolation of deacetoxydialdehydic ligstroside aglycone “Oleocanthal”A. Synthesis of Oleocanthal
(5) Initially (+)- and (-)-cyclopentanones (10) were prepared via the sulfoximine and/or enzymatic protocols introduced and developed by Johnson (Johnson, C.R. and T. Penning (1988) J. Am. Chem. Soc. 110:4726-4735; Johnson, C.R. (1998) Acc. Chem. Res. 31:333-341). Although effective on modest scale (10-100mg), the requirement for gram quantities of the oleocanthals demanded that we secure for more scalable routes to (10). Towards this end, we optimized a hybrid of synthetic approaches (Moon, H. et al. (2002) Tetrahedron: Asym. 13(11):1189-1193; Jin, Y. et al. (2003) J. Org. Chem. 68(23):9012-9018; Yang, M. (2004) J. Org. Chem. 69(11):3993-3996; Palmer, A. et al. (2001) Eur. J. Org. Chem. 66(7):1293-1308; Paquette, L. and S. Bailey (1995) J. Org. Chem. 60:7849-7856) as outlined in Scheme 2. Importantly, both enantiomers of (10) could be prepared in multi-gram quantities in 7 steps, with an overall efficiency of 40% from inexpensive D-(-)-ribose. Key elements of both sequences entailed vinyl Grignard addition to the enantiomers of aldehyde (12), followed in turn by ring closing metathesis (RCM), PCC oxidation and hydrogenation (Scheme 2).





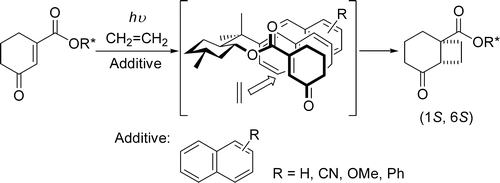
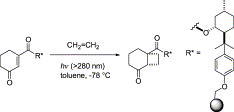
The diastereoselective [2+2] photocycloaddition of ethylene to a chiral cyclohexenone was studied in a continuous flow microcapillary reactor. In all cases examined, the microcapillary reactor gave higher conversions and selectivity than the batch system, even after shorter irradiation times. These findings were explained by the superior temperature control, favorable light penetration, and generation of a gas–liquid slug flow with improved mass transfer in the microreactor.
http://www.akademiai.com/content/03163u0p80225v14/?p=bb18d4ec7c044f5c80013806493e8850&pi=2
| Journal of Flow Chemistry | |
| Publisher | Akadémiai Kiadó |
| ISSN | 2062-249X (Print) 2063-0212 (Online) |
| Subject | Flow Chemistry |
| Issue | Volume 2, Number 3/September 2012 |
| Pages | 73-76 |
| DOI | 10.1556/JFC-D-12-00005 |
kakiuchi@ms.naist.jp, http://mswebs.naist.jp/LABs/kakiuchi/member/staff/CV_kakiuchi.pdf
1Nara Institute of Science and Technology (NAIST) Graduate School of Materials Science 8916-5 Takayama-cho, Ikoma Nara 630-0192 Japan
2James Cook University School of Pharmacy and Molecular Sciences Townsville QLD 4811 Australia
more………..
http://mswebs.naist.jp/LABs/kakiuchi/achevement/paper.htm
“Novel Enhancement of Diastereoselectivity of [2+2] Photocycloaddition of
Chiral Cyclohexenones to Ethylene by Adding Naphthalenes”
Ken Tsutsumi, Hiroaki Nakano, Akinori Furutani, Katsunori Endou, Abdurshit Merpuge
Takuya Shintani, Tsumoru Morimoto, Kiyomi Kakiuchi
J. Org. Chem. 2004, 69, 3, 785-789.
“Diastereoselective [2+2] Photocycloaddition of Polymer-Supported
Cyclic Chiral Enone with Ethylene”
Takuya Shintani, Kazunori Kusabiraki, Atsuko Hattori, Akinori Furutani, Ken Tsutsumi,
Tsumoru Morimoto, Kiyomi Kakiuchi
Tetrahedron Lett. 2004, 45, 9, 1849-1851.
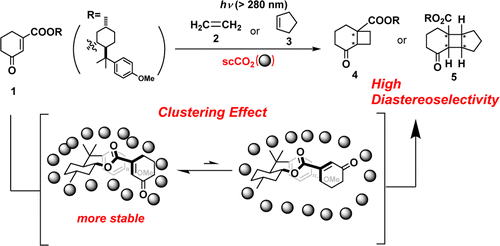
“Diastereoselective [2+2] Photocycloaddition of Cyclohexenone Derivative with Olefines in Supercritical Carbon Dioxide”
Yasuhiro Nishiyama, Kazuya Nakatani, Hiroki Tanimoto, Tsumoru Morimoto, Kiyomi Kakiuchi
J. Org. Chem. 2013, 78, 7186-7193.
Highlighted in
ChemInform 2013, 44(44)
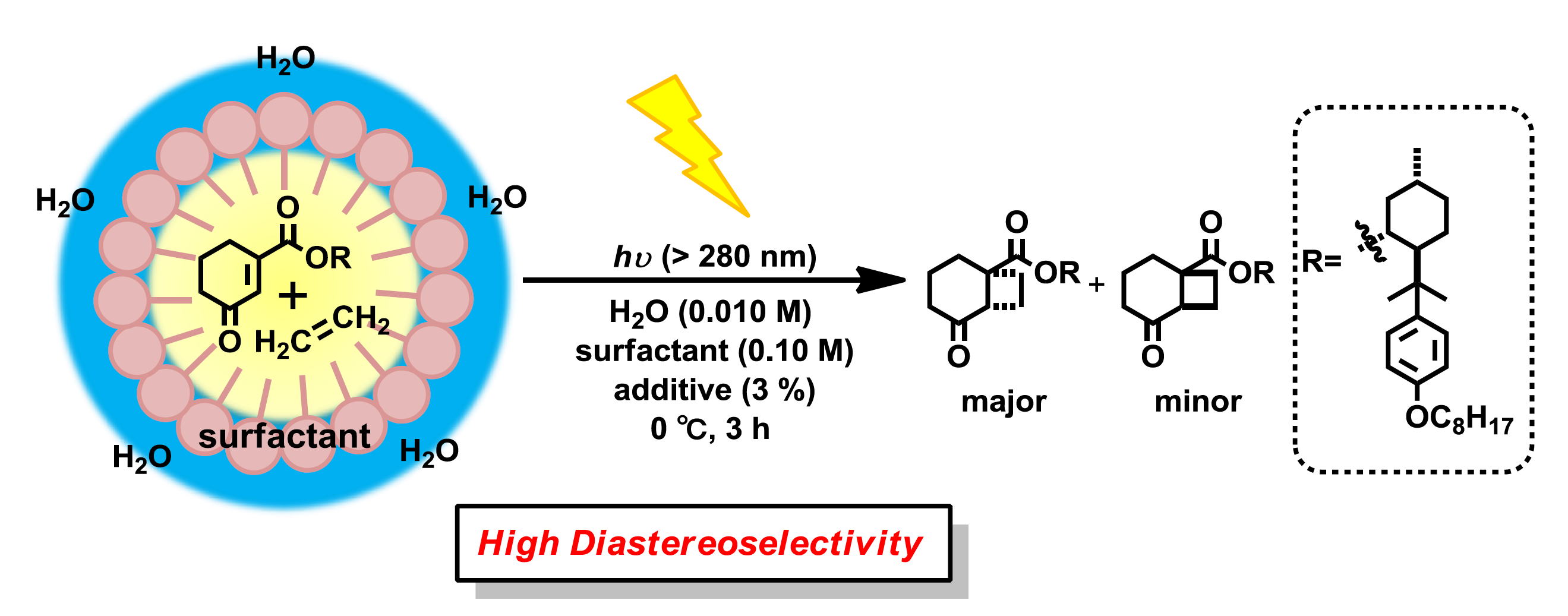
“Diastereoselective [2+2] Photocycloaddition of Chiral Cyclic Enones with Olefins in Aqueous Media Using Surfactants”
Yasuhiro Nishiyama, Mikiko Shibata, Takuya Ishii, Tsumoru Morimoto, Hiroki Tanimoto,
Ken Tsutsumi, Kiyomi Kakiuchi
Molecules, 2013, 18, 1626-1637.
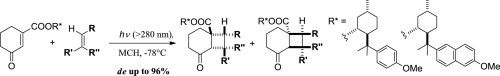
“Highly diastereodifferentiating and regioselective [2+2]-photoreactions using methoxyaromatic menthyl cyclohexenone carboxylates”
Inga Inhulsen, Naoya Akiyama, Ken Tsutsumi, Yasuhiro Nishiyama, Kiyomi Kakiuchi
Tetrahedron 2013, 69, 782-790.
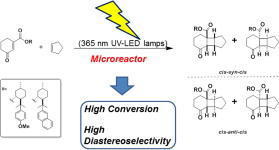
“Diastereodifferentiating [2+2] Photocycloaddition of Chiral Cyclohexenone Carboxylates with Cyclopentene by a Microreactor”
Kimitada Terao, Yasuhiro Nishiyama, Shin Aida, Hiroki Tanimoto, Tsumoru Morimoto,
Kiyomi Kakiuchi
J. Photochem. Photobiol. A: Chem. 2012, 242, 13-19.

http://pubs.rsc.org/en/Content/ArticleLanding/2015/OB/C4OB02376E#!divAbstract
DOI: 10.1039/C4OB02376E
Dr. David Balcells, Prof. Odile Eisenstein, Prof. Robert H Crabtree, Agusti Lledos Departament de Quimica, Universitat Autonoma de Barcelona, Bellaterra, Spain; Institut Charles Gerhardt, Universite Montpellier 2, Montpellier, France; Department of Chemistry, Yale University, New Haven, United States
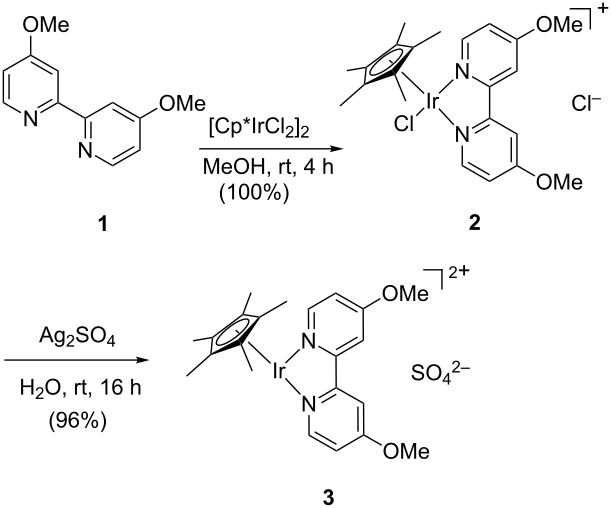
The development of a new energy model is a major challenge in modern chemistry. The climate change and the raise of oil prices prompt the development of clean and cheap energy resources. In this field, artificial photosynthesis is one of the most promising solutions.1 The catalytic oxidation of water to dioxygen is a fundamental part of this process. The mononuclear iridium complex Cp*Ir(ppy)(Cl) (ppy = phenylpyridine) is one of the most efficient catalysts reported for this reaction (Figure).2 DFT calculations support the oxo complex Cp*IrO(ppy) as the active species. The electronic structure of this complex is characterized by having the antibonding p*(Ir=O) orbitals half-occupied. The calculations suggest that the reaction mechanism consists of an intermolecular attack of water to the oxo ligand. This reaction involves the formation of the O-O bond and a proton transfer, which is assisted by the molecules of water solvating the catalyst.

Figure. Iridium-catalyzed water oxidation.
References
(1) Hammarström, L.; Hammes-Schiffer, S. Acc. Chem. Res. 2009, 42, 1859-1860.
(2) Hull, J. F.; Balcells, D.; Blakemore, J. D.; Incarvito, C. D.; Eisenstein, O.; Brudvig, G. W.; Crabtree, R. H. J. Am. Chem. Soc.2009, 131, 8730-8731.
Article first published online: 13 JAN 2014
DOI: 10.1002/adsc.201300711
http://onlinelibrary.wiley.com/doi/10.1002/adsc.201300711/abstract
http://www.beilstein-journals.org/bjoc/single/articleFullText.htm?publicId=1860-5397-9-110

6-[(R)-Amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone;
(R)-(+)-R 115777; Zarnestra; 192185-68-5
zarnestra, 192185-72-1, R115777, R-115777, IND 58359, UNII-MAT637500A
192185-72-1, 192185-68-5
Tipifarnib (trade name Zarnestra) is a farnesyltransferase inhibitor that is being investigated in patients 65 years of age and older with newly diagnosed acute myeloid leukemia (AML). It inhibits the Ras kinase in a post translational modification step before the kinase pathway becomes hyperactive. It inhibits prenylation of the CxxX tail motif, which allows Ras to bind to the membrane where it is active. Without this step the protein cannot function.
It is also being tested in clinical trials in patients in certain stages of breast cancer.[1]
For treatment of progressive plexiform neurofibromas associated with Neurofibromatosis type I, it successfully passed phase one clinical trials but was suspended (NCT00029354) in phase two.[2][3] The compound was discovered by and is under investigation byJohnson & Johnson Pharmaceutical Research & Development, L.L.C, with registration number R115777.
Tipifarnib was submitted to the FDA by Johnson & Johnson for the treatment of AML in patients aged 65 and over with a New Drug Application (NDA) to the Food and Drug Administration (FDA) on January 24, 2005.
In June 2005, the FDA issued a “not approvable” letter for tipifarnib.[4]
Farnesyltransferase inhibitors block the main post-translational modification of the Ras protein, thus interfering with its localization to the inner surface of the plasma membrane and subsequent activation of the downstream effectors. Although initially developed as a strategy to target Ras in cancer, farnesyltransferase inhibitors have subsequently been acknowledged as acting by additional and more complex mechanisms that may extend beyond Ras involving GTP-binding proteins, kinases, centromere-binding proteins and probably other farnesylated proteins.
A particular farnesyltransferase inhibitor is described in WO 97/21701, namely (R)-(+)-6-[amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone. The absolute stereochemical configuration of the compound was not determined in the experiments described in the above-mentioned patent specification, but the compound was identified by the prefix “(B)” to indicate that it was the second compound isolated from column chromatography. The compound thus obtained has been found to have the (R)-(+)-configuration. This compound will be referred to below by its published code number R115777 and has the following formula (V).
R115777 (Tipifarnib) is a potent, orally active inhibitor of farnesylprotein transferase. It is one of the most advanced of the farnesylprotein transferase inhibitors currently reported to be in clinical development, being one of the agents that have progressed to phase III studies.
R115777 has been found to have very potent activity against neoplastic diseases. Antineoplastic activity in solid tumors, such as breast cancer, as well as in haematological malignancies, such as leukemia, have been observed. Also combination studies have been carried out demonstrating that R115777 can be safely combined with several highly active anticancer drugs.
In WO 01/53289, the racemates (±) (4-(3-chloro-phenyl)-6-[(6-chloro-pyridin-3-yl)-(4-methoxy-benzylamino)-(3-methyl-3H-imidazol-4-yl)-methyl]-1-cyclopropylmethyl-1H-quinolin-2-one (racemate 1) and (±) 4-(3-chloro-phenyl)-6-[(6-chloro-pyridin-3-yl)-[(4-methoxy-benzylidene)-amino]-(3-methyl-3H-imidazol-4-yl)-methyl]-1-cyclopropylmethyl-1H-quinolin-2-one (racemate 2) are prepared.
After chiral molecule separation using column chromatography, either the benzylamino or the benzilidine moiety of the resulting (+) and/or (−) enantiomers are converted to an amino group under acidic conditions.
In WO 97/21701, it is described (on page 9, line 7-14) that intermediates of formula (XIII), can be prepared by reacting an intermediate of formula (XIV), wherein W is an appropriate leaving group, such as, for example, halo, with an intermediate ketone of formula (XV). In WO 97/21701, it is described that this reaction can be performed by converting the intermediate of formula (XV) into an organometallic compound, by stirring it with a strong base such as butyl lithium and subsequently adding the intermediate ketone of formula (XV). It is further indicated that although this reaction gives at first instance a hydroxy derivative (i.e. R8 is hydroxy), said hydroxy derivative can be converted into other intermediates wherein R8 has another definition by performing art-known (functional group) transformations. The drawings of the compounds of formula (XIII), (XV) and (XIV) have been taken over from WO 97/21701 and the substituents in these drawings are as defined in WO 97/21701.
In WO 97/21701, it is also described (from page 7 line 32, to page 8 line 6) that the compounds of formula (XVI), wherein R is C1-6alkyl, R(2-8, 16-19) can be a substituent chosen from lists as defined in WO 97/21701 and R1 has a meaning as defined in WO 97/21701 apart from hydrogen, may be prepared by hydrolysing an intermediate ether of formula (XIII), according to art-known methods, such as stirring the intermediate of formula (XIII) in an aqueous acid solution. An appropriate acid can be for instance hydrochloric acid. Subsequently the resulting quinolinone, wherein R1 is hydrogen, may be transformed into a quinolinone of formula (XVI) by art-known N-alkylation. The drawings of the compounds of formula (XIII) and (XVI) have been taken over from WO 97/21701 and the substituents in these drawings are as defined in WO 97/21701.
The synthesis of R115777 as originally described in WO 97/21701, is presented in scheme 1.
Herein, in step 1, the intermediate 1-methyl imidazole in tetrahydrofuran, is mixed with a solution of n-butyllithium in a hexane solvent to which is added chlorotriethylsilane (triethylsilyl chloride), followed by a further addition of n-butyllithium in hexane, the resulting mixture being cooled to −78° C. before the addition of a solution of a compound of formula (I), i.e. 6-(4-chlorobenzoyl)-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone in tetrahydrofuran. The reaction mixture is subsequently brought to room temperature, and then hydrolysed, extracted with ethyl acetate and the organic layer worked up to obtain a compound of formula (II), i.e. (±)-6-[hydroxy(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone.
In step 2, the hydroxy compound of formula (II) is chlorinated with thionylchloride to form a compound of formula (III), i.e. (±)-6-[chloro(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone.
In step 3, the chloro compound of formula (III) is treated, with NH4OH in tetrahydrofuran to form the amino compound of formula (IV), i.e. (±)-6-[amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone.
In step 4, the amino compound of formula (IV) is separated into its enantiomers by chiral column chromatography over Chiracel OD (25 cm; eluent: 100% ethanol; flow: 0.5 ml/min; wavelength: 220 nm). The pure (B)-fractions are collected and recrystallised from 2-propanol resulting in R115777, the compound of formula (V).
However, the procedure described in WO97/21701 has a number of disadvantages. For example, during the first step, the procedure results in the undesired formation of a corresponding compound of formula (XI), i.e. 6-[hydroxy(4-chlorophenyl)(1-methyl-1H-imidazol-2-yl)methyl]-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone), in which the imidazole ring is attached to the remainder of the molecule at the 2-position of the ring, instead of the desired 5-position. At the end of the procedure, this results in the formation of a compound of formula (XII), i.e. 6-[amino(4-chlorophenyl)(1-methyl-1H-imidazol-2-yl)methyl]-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone.
Furthermore, the purification of compound (V) using chiral chromatography is expensive and disadvantageous in view of the large amounts of solvent needed and the specialised equipment required to perform a large scale chiral chromatography.
Another process for the synthesis of R115777 as described in WO 02/072574, is presented in scheme 2.
Herein, in step 1, 1-methyl imidazole in tetrahydrofuran is mixed with a solution of n-hexyllithium in a hexane solvent to which is added tri-iso-butylsilyl chloride, followed by a further addition of n-hexyllithium in hexane. The compound of formula (I) in tetrahydrofuran is then added to the reaction mixture, keeping the temperature between −5° C. and 0° C. The resulting product of formula (II) is isolated by salt formation.
In step 2, the chlorination reaction is effected by treatment of the compound of formula (II) with thionyl chloride in 1,3-dimethyl-2-imidazolidinone.
In step 3, the chloro compound of formula (III) is treated with a solution of ammonia in methanol. After the addition of water, the compound of formula (IV), precipitates and can be isolated.
In step 4, the compound of formula (IV) can be reacted with L-(−)-dibenzoyl tartaric acid (DBTA) to form the diastereomeric tartrate salt with formula (VI) i.e. R-(−)-6-[amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone [R—(R*,R*)]-2,3-bis(benzoyloxy)butanedioate (2:3).
Finally, in step 5, the compound of formula (VI) is treated with aqueous ammonium hydroxide, to form the crude compound of formula (V) which is then purified by recrystallisation from ethanol to the pure compound (V).
| Patent | Submitted | Granted |
|---|---|---|
| Process for the preparation of imidazole compounds [US6844439] | 2004-07-15 | 2005-01-18 |
| TREATMENT OF MITOCHONDRIAL DISORDERS USING A FARNESYL TRANSFERASE INHIBITOR [US2010331363] | 2010-12-30 | |
| TREATMENT OF MITOCHONDRIAL DISORDERS USING A FARNESYL TRANSFERASE INHIBITOR [US2011060005] | 2011-03-10 | |
| Diastereoselective Synthesis Process with 6-Bromo-4-(3-Chlorophenyl)-2-Methoxy-Quinoline [US7572916] | 2007-12-20 | 2009-08-11 |
| Anti-cancer phosphonate analogs [US7452901] | 2006-04-13 | 2008-11-18 |
| Diastereoselective Synthesis Process for the Preparation of Imidazole Compounds [US7456287] | 2007-10-11 | 2008-11-25 |
| Diastereoselective Addition of Lithiated N-Methylimidazole on Sulfinimines [US7524961] | 2007-12-20 | 2009-04-28 |
| Therapeutic phosphonate compounds [US7645747] | 2006-11-23 | 2010-01-12 |
| TREATMENT OF PROTEINOPATHIES USING A FARNESYL TRANSFERASE INHIBITOR [US2010160372] | 2010-06-24 | |
| ANTI-CANCER PHOSPHONATE ANALOGS [US2010022467] | 2010-01-28 |
Ti(OEt)4 (0.0162 mol) was added to a mixture of (4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methanone (0.0032 mol) and (R)-(+)-2-methyl-2-propane-sulfinamide (0.0032 mol) in DCE (7 ml). The mixture was stirred and refluxed for 6 days, then cooled to room temperature. Ice water was added. The mixture was filtered over celite. Celite was washed with DCM. The organic layer was extracted with saturated sodium chloride. The organic layer was separated, dried (MgSO4), filtered, and the solvent was evaporated. This fraction was purified by column chromatography over silica gel (40 μm) (eluent: DCM/MeOH/NH4OH 97/3/0.5), yielding 0.475 g of compound 25 (46%).
The compound N-[(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methylene)]-2-methyl-2-propanesulfinamide [(S(S)] can be obtained in an analogous way.
b) Preparation of N-[(4-chlorophenyl)((4-(3-chlorophenyl)-2-methoxy-quinoline-6-yl)(1-methyl-1H-imidazole-5-yl)methyl]-2-methyl-2-propanesulfinamide [S(R)] (Compound 26)
n-Butyllithium (0.00081 mol) in hexane, was added dropwise at −78° C. to a mixture of 6-bromo-4-(3-chlorophenyl)-2-methoxy-quinoline (0.00081 mol) in THF (3 ml) under nitrogen flow. The mixture was stirred at −78° C. for 30 minutes. A solution of compound 25 (0.00065 mol) in THF (0.6 ml) was added. The mixture was stirred at −78° C. for 1 hour and 30 minutes, poured out into ice water and extracted with EtOAc. The organic layer was separated, dried (MgSO4), filtered, and the solvent was evaporated. This fraction was purified by column chromatography over silica gel (40 μm)(eluent: DCM/MeOH/NH4OH 97/3/0.1). The pure fractions were collected and the solvent was evaporated, yielding 0.138 g (36%) of compound 26, melting point 153° C.
The compound N-[(4-chlorophenyl)((4-(3-chlorophenyl)-2-methoxy-quinoline-6-yl)(1-methyl-1H-imidazole-5-yl)methyl]-2-methyl-2-propanesulfinamide [S(S)] can be obtained in an analogous way
c) Preparation of (S)-1-(4-chlorophenyl)-1-[4-(3-chlorophenyl)-2-methoxy-quinoline-6-yl]-1-(1-methyl-1H-imidazole-5-yl)-methylamine (Compound 27)
Hydrochloric acid in isopropanol was added to a solution of compound 26 (0.000018 mol) in methanol (4.2 ml). The mixture was stirred at room temperature for 30 minutes. The mixture was added to potassium carbonate (10%) on ice and extracted with ethyl acetate. The organic layer was separated, washed with a solution of saturated sodium chloride, dried (MgSO4), filtered, and evaporated giving 0.086 g (100%) of compound 27, melting point 96° C., enantiomeric excess 88%.
d) Preparation of (S)-6-[amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1H)-quinolin-2-one (Compound 28)
Compound 27 (0.00038 mol) in hydrochloric acid 3N (9.25 ml) and THF (9.25 ml), was stirred at 60° C. for 24 hours and evaporated, giving 0.18 g (100%) of compound 28, melting point 210° C.
EXAMPLE A.2 a) Preparation of N-[(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methylene)]-p-toluenesulfinamide [(S(S)](Compound 29)
Ti(OEt)4 (0.0419 mol) was added to a mixture of (4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methanone (0.0084 mol) and (S)-(+)-p-toluenesulfinamide (0.0084 mol) in DCE (18 ml). The mixture was stirred and refluxed for 7 days, then cooled to room temperature. Ice water was added. The mixture was filtered over celite. Celite was washed with DCM. The organic layer was extracted with saturated sodium chloride. The organic layer was separated, dried (MgSO4), filtered, and the solvent was evaporated. This fraction was purified by column chromatography over silica gel (40 μm) (eluent: DCM/MeOH/NH4OH 97/3/0.5), yielding 1.15 g of compound 29 (38%).
The compound N-[(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methylene)]-p-toluenesulfinamide [(S(R)] can be obtained in an analogues way.
B. Preparation of Final Compounds
EXAMPLE B.1 a) Preparation of (S)-6-[amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone (Compound 30)
Compound 28 (0.00038 mol) was added to a solution of THF (1.8 ml) and NaOH 10N (1.8 ml). BTEAC (0.0019 mol) and methyliodide (0.00076 mol) were added and the mixture was stirred for 2 hours at room temperature. EtOAc was added. The organic layer was separated, dried (MgSO4), filtered, and evaporated giving 0.149 g (83%) of compound 30, enantiomeric excess 86%.
Angibaud, P.; Venet, M.; Filliers, W.; Broeckx, R.; Ligny, Y.; Muller, P.;Poncelet, V.; End, D. Eur. J. Org. Chem. 2004, 479.
see………….http://onlinelibrary.wiley.com/doi/10.1002/ejoc.200300538/abstract
(b) Filliers, W.; Broeckx, R.;Angibaud, P. U.S. patent, US7572916, 2009.
Scaling from Milligrams to 1-2Kg
see at…………..http://news.scientificupdate.co.uk/index.php?action=social&chash=1c1d4df596d01da60385f0bb17a4a9e0.1201
2 – 3 March 2015
Sheraton Fisherman’s Wharf Hotel - San Francisco

The aim of this professional development course is to provide a good basis to work from when involved in taking development candidates to the first in human trials and with a view on some longer-term requirements. The course content will focus on the necessary early phases of chemical development, as would typically be required to support production of up to about 2kg.
The course will introduce and discuss the following:

|
|
|
|
|




Methohexital or methohexitone, (marketed under the brand name Brevital) is a drug which is a barbiturate derivative. It is classified as short-acting, and has a rapid onset of action. It is similar in its effects to sodium thiopental, a drug with which it competed in the market for anaesthetics.
Methohexital binds to a distinct site which is associated with Cl− ionophores at GABAA receptors.[1] This increases the length of time which the Cl− ionopores are open, thus causing an inhibitory effect.
Metabolism of methohexital is primarily hepatic (i.e., taking place in the liver) via demethylation and oxidation.Side-chain oxidation is the primary means of metabolism involved in the termination of the drug’s biological activity.
Protein binding is approximately 73% for methohexital.
Methohexital is primarily used to induce anesthesia, and is generally provided as a sodium salt (i.e. methohexital sodium). It is only used in hospital or similar settings, under strict supervision.[citation needed] It has been commonly used to induce deep sedation or general anesthesia for surgery and dental procedures. Unlike many other barbiturates, Methohexital actually lowers the seizure threshold, a property that make it particularly useful when anesthesia is provided for a electroconvulsive therapy (ECT). And rapid recovery rate with consciousness being gained within three to seven minutes after induction and full recovery within 30 minuntes is a major advantage over other ECT barbiturates (Schulgasser and Borowitz 1963).
Methohexital, 5-allyl-1-methyl-5-(1-methyl-2-pentinyl barbituric acid, is synthesized in the classic manner of making barbituric acid derivatives, in particular by the reaction of malonic ester derivatives with derivatives of urea.
Methohexital synthesis: W.J. Doran, U.S. Patent 2,872,448 (1959).
The resulting allyl-(1-methyl-2-pentynyl) malonic ester is synthesized by subsequent alkylation of the malonic ester itself, beginning with 2-bromo-3-hexyne, which gives (1-methyl-2-pentynyl)malonic ester, and then by allylbromide. In the final step, reaction of the disubstituted malonic ester with N-methylurea gives desired methohexital.

 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
| 5-hex-3-yn-2-yl-1- methyl-5-prop-2-enyl-1, 3-diazinane-2,4,6-trione | |
| Clinical data | |
| AHFS/Drugs.com | Consumer Drug Information |
|
|
| Legal status |
|
| Routes | Intravenous, rectal |
| Pharmacokinetic data | |
| Bioavailability | I.V. ~100% Rectal ~17% |
| Metabolism | Hepatic |
| Half-life | 5.6 ± 2.7 minutes |
| Excretion | ? |
| Identifiers | |
| CAS number | 151-83-7  |
| ATC code | N01AF01 N05CA15 |
| PubChem | CID 9034 |
| DrugBank | DB00474 |
| ChemSpider | 8683  |
| UNII | E5B8ND5IPE  |
| KEGG | D04985  |
| ChEBI | CHEBI:102216  |
| ChEMBL | CHEMBL7413  |
| Chemical data | |
| Formula | C14H18N2O3 |
| Molecular mass | 262.304 |

http://www.gccpo.org/DefaultEn.aspx

The Patent Office of the Cooperation Council for the Arab States of the Gulf is delighted to announce launching the electronic filing system for patent applications “Protection” in its trial version, starting from the second of August of 2014, through the office’s official website on the internet www.gccpo.org.
The “Protection” system launch comes within the frame of the office’s comprehensi…More
The GCC Patent Office (GCCPO) is a regional patent office based in Riyadh, Saudi Arabia, within the Secretariat General of the Gulf Cooperation Council (GCC). It was established in 1992 and began operations in 1998. The GCC Patent Office grants patents valid in all GCC member states. The first GCC patent was granted in 2002. As of 2013, it employed about 30 patent examiners.[1]
Mizael Al-Harbi, Hussam Al-Muqhim (18 April 2013). “Gulf countries: introduction to the GCC patent system”. East meets West 2013. Austria.
The Gulf Cooperation Council Patent Office (GCC Patent Office) is a unified patent office that serves as a convention patent office for the states of the Gulf namely, Saudi Arabia, Bahrain, Kuwait, Oman, Qatar and United Arab Emirates. The GCC Patent Office became operational in 1998. Saudi Arabia is a member of the Gulf Cooperation Council. A patent issued by the GCC Patent Office is valid and enforceable in all GCC States.
Under GCC Patent Law, it is possible to claim priority from an earlier foreign application. As compared with the Saudi Patent Office the examination process is much faster in the GCC Patent Office. Moreover, the GCC Patent Office has the following advantages:
The requirements for filing a new GCC patent application are as follows:


Industrial property is one of the branches of intellectual property. It refers to the protection of rights related to the creations of the mind applied in industrial, commercial, and agricultural fields. It covers:
• Inventions
• Industrial Designs
• Plant Varieties
• Layout Designs of integrated circuits
• Trademarks
• Geographical indications of source
King Abdulaziz City for Science and Technology (KACST) grants protection documents for inventions, layout designs of integrated circuits, plant varieties and industrial designs.
History of Industrial Property in Saudi Arabia.
In 1982, KACST was notified of the Royal Decree approving the accession of Saudi Arabia to the World Intellectual Property Organization (WIPO). Accordingly, patents has been assigned to KACST since intellectual property is essentially about patents and technology transfer and KACST is the scientific body qualified for this mission.
The first Law of Patents was issued by the Royal Decree No. (M / 38) dated 10/6/1409 H (18/1/1989 AD). The Law aimed to provide protection for inventions in Saudi Arabia, and it was modified in 19 / 7 / 1425 H (4/9/2004 AD).
The modified Law of Patents, Layout Designs of Integrated Circuits, Plant Varieties, and Industrial Designs was issued by the Royal Decree No. (M/27) dated 17/7/2004 and was published in the Official Gazette (Om Alqura) in 7/8/2004, and became effective as of 5/9/2004.
Kingdom of Saudi Arabia
Patent Application Flowchart

Desgin Application Flowchart

Saudi Arabia has ratified the Berne Convention for the Protection of Literary and Artistic Works of 1886, revised in Paris on 24th July 1971 and the Paris Convention for the Protection of Industrial Property of 1883, both with effect from 11th March 2004. Three government authorities have authority to protect and enforce intellectual property rights: the Ministry of Commerce and Industry for trademarks, the Ministry of Culture and Information for copyright, and King Abdulaziz City for Science and Technology for patents.
Trademarks:
Trademarks are governed by the Trademarks Regulation, Royal Decree No. M/21 of 28th Jumada Awal 1423 Hejra corresponding to 8th August 2002 Gregorian, and its Implementing Rules of the same year. Applications for registration must be made to the Trademarks Office of the Ministry of Commerce and Industry which applies the ‘Nice Classification’ in accordance with the Agreement Concerning the International Classification of Goods and Services for the Purposes of the Registration of Marks of 1957.
Applications must contain the following particulars:
01. A copy of the trademark required to be registered.
02. Name, title, address, nationality and trade name of the applicant (if any). If the applicant is a juristic person, the name, address of the head office and nationality must be stated.
03. Where the application is submitted by an attorney, his name, title and address must be stated.
04. Description of the trademark required to be registered.
05. The products or services in respect of which the trademark is required to be registered, and the classification thereof.
06. Signature of the applicant or the attorney thereof.
07. Ten representations of the trademark identical to the trademark sample shown in the application for registration.
08. A copy of the power of attorney together with the original for verifying purposes must be attached where the application was submitted by an attorney of the person concerned.
09. Evidence of payment of application fees as stipulated in the Trademarks Regulation.
It is not permitted to register in Saudi Arabia, by other than its rightful owner, a trademark that is similar to an internationally known mark. Registration of a trademark allows holders protection for ten years from the date of application, renewable for similar periods. Any renewal must be specifically applied for before the end of the last year of expiry of the registration, and the procedure for renewal is the same as the one for the initial registration of the trademark. Service marks are included in the definition of trademarks. A trademark is deemed owned by the person who effects the registration. Once the registration is effected in the trademarks register, the party who has registered the trademark shall be considered the owner thereof to the exclusion of others.
A trademark can be licensed, pledged or transferred by the rightful owner. The trademark may be deleted or cancelled if it is not used for five consecutive years. Penalties for infringement of a valid trademark include imprisonment for a period of not more than one year and a fine of not less than SR50,000 and not more than SR1,000,000. Any civil or criminal disputes arising from the infringement are settled by the Board of Grievances.
Patents:
There are at present two overlapping patents systems in Saudi Arabia. The GCC Patents of Inventions Regulation of 2001, which is an amendment of an earlier statute of 1992, was approved in Saudi Arabia by Royal Decree No. M/28 of 2001. This permits the registration of patents with effect throughout the GCC countries. The GCC Patent Office is based in Riyadh.
Under Saudi Arabian law, patents are governed by the Layout Designs of Integrated Circuits, Plant Varieties, and Industrial Models Regulation, Royal Decree No. M/27 of 20th Jumada Awal 1425 Hejra corresponding to 17th July 2005 Gregorian, which gives effect to the Paris Convention for the Protection of Industrial Property under Saudi Arabian domestic law.
A protection document is granted by the General Directorate of Patents at King Abdulaziz City for Science and Technology, which gives full protection within the Kingdom to an invention, a layout design of an integrated circuit, a plant variety, or an industrial design. The protection document grants the owner the right to commercially exploit the subject matter of protection.
Applications for a protection document must be filed at the Directorate in the Arabic language, and must include:
01. Names and addresses of the applicant(s) and inventor(s);
02. Name and address of the local agent and the authorization, if the applicant resides outside the Kingdom;
03. A brief title of the subject matter of the application, an original copy and certified copies of the complete specification and certified copies of other relevant details thereof like examination and research reports;
04. Priority and disclosure information including previous filings; and
05. Evidence of payment of the filing fee at a designated bank, stipulated by the Directorate.
The protection document is the personal right of the owner and he may transfer or assign it or grant a contractual licence to others to commercially exploit the subject matter of protection. Protection is granted to the owner for a duration of 20 years for an invention, 10 years for an industrial design and a layout design of an integrated circuit, and 20 to 25 years for a new plant variety. The above periods are renewable, for an annual fee.
Copyright
The Copyright Regulation, Royal Decree No. M/41 of 2nd Rajab 1424 Hejra corresponding to 30th August 2003 Gregorian and its Implementing Rules, Resolution of the Minister of Commerce and Industry No. M/W/1788/1 of 10th Rabi Thani 1425 Hejra corresponding to 30th May 2004 Gregorian, define copyright protection to include architectural designs, speeches, theatrical, musical, photographic and cinematographic works, as well as works for radio and television, maps, video tapes and computer software. Copyright protection is not subject to any registration or renewal. The Regulation gives the author financial and moral rights, to print or publish the work, to make amendments or to delete his work, to withdraw it from circulation, and to assign it as he wishes.
In general the duration of protection afforded to different types of Copyright works is as follows:
01. The period of protection of copyright for the author of a work shall be for the duration of his life and for a period of fifty years following his death.
02. The period of protection for works where the author is a corporate entity, or if the author’s name is unknown, shall be fifty years from the date of the first publication of the work.
03. The protection period for sound works, audio-visual works, films, collective works and computer programs is fifty years from the date of the first show or publication of the work, regardless of republication.
04. The protection period for applied art (handcrafted or manufactured) and photographs shall be twenty-five years from the date of publication.
05. The protection period for broadcasting organizations shall be twenty years from the date of the first transmission of program or broadcast materials.
A special Copyright Violations Committee under the authority of the Ministry of Culture and Information presides over copyright infringement issues and it has broad powers to punish the infringer of a valid copyright including a fine of up to SR250,000 in the case of first time offenders, and this can be raised to SR500,000 if there is repeated infringement. The Committee may issue injunctions in certain cases and also order imprisonment of an offender. Any decision of the Violations Committee can be appealed by filing a claim with the Board of Grievances.
 kacst
kacst
Saudi Arabia is the largest and richest of the Gulf States. Its wealth derives mainly from its vast reserves of oil and natural gas, which places the country as the largest exporter of petroleum and give it a leading role in the Organization of the Petroleum Exporting Countries. It possesses about one-fifth of the world’s proven petroleum reserves. The Intellectual Property framework of Saudi Arabia has evolved to protect the different intellectual properly rights and to cope with the rapid globalization and technological change. Both globalization and technological advancement have presented significant economic opportunities and challenges to the IP system. As a result, IP rights have become increasingly important in the country, but many of the challenges facing the IP system have yet to be addressed.
The Patent Office in Saudi Arabia is located at the King Abdulaziz City for Science & Technology, an independent scientific organization of the Saudi Arabian Government established back in the year 1977. The Patent Office’s main activities and objectives are to:
(1) Apply the patent law and its implementing regulations: Law of Patents, Layout Designs of Integrated Circuits, Plant Varieties and Industrial Designs
(2) Grant Saudi patents, Layout Designs of Integrated Circuits, Plant Varieties and Industrial Designs
(3) Establish a Registry for the collection of local and foreign Layout Designs of Integrated Circuits, Plant Varieties and Industrial Designs
(4) Publish the Patents Gazette
(5) Encourage the inventiveness of Saudi nationals.
The Office has taken a number of measures during the past years to hire and train examiners and translators in order to adequately handle patent applications.
Legislation
Law no. 159 on the Protection of Patents, Layout Designs of Integrated Circuits, Plant Varieties and Industrial Models.
Types of Patents
- Patents of Invention
- Divisional Patents
Priority Claim
Saudi Arabia is a member of the Paris Convention. Applicants can benefit from a right of priority of 12 months to file a corresponding Saudi patent application.
Patent Cooperation Treaty
Saudi Arabia has joined the PCT but still the entry of national phase of PCT application is not possible as the relevant regulations and laws have not been issued, we expect the implementation of PCT within next 6-12 months.
Definition of an Invention
An invention must be novel, involves an inventive step and capable of industrial application. The invention may be a product, an industrial process or relates to either (Article 43).
Types of Claims
Product and process claims are acceptable. When a patent is granted for a process, any product made directly by such a process is also protected (Article 47(b)).
Exception to Protection
An invention is not patentable if it is: a discovery; a scientific theory or mathematical method; an aesthetic creation such as a literary, dramatic, or artistic work; a scheme or method for performing a mental act, games or business methods; the presentation of information; or a computer program.
Examination
- Novelty: the invention must never have been made public in any way, anywhere in the world, before the filing date or the priority date.
- Inventiveness: an invention involves an inventive step if, when compared with what is already known, it would not be obvious to someone skilled in the relevant field.
- Industrial applicability: an invention must be capable of being made or used in some kind of industry.
Novelty
Absolute novelty is required. Any act that makes an invention available to the public before the filing date or priority date has the effect of barring the invention from being patented in Saudi Arabia (Absolute Novelty). Examples of acts that can make an invention available to the public are publications, sales, public oral disclosures and public demonstrations or use, etc. However, the patent application can still be filed if disclosure was very limited and can not be cited by the examiner. Also, a one year grace period is available if the disclosure of the invention was proven to be made without the knowledge or consent of the inventor (Article 44). Wrong statement article 44 if disclosed within the priority period, but the exception is under article 30 of Implementing Regulations where the grace period is 6 months if the disclosure occurred because of abusive action.
Application Workflow
A formal examination is conducted first. If application fails to meet all the set requirements, the applicant will be notified and will be given a period of 90 days to complete the application. Once completed, the application will proceed to substantive examination. The examiner will assess the application for patentability (novelty and industrial applicability). If the claimed invention is not patentable, the applicant will be requested to present counter-arguments within 90 days from notification date. If the claimed invention is patentable, the applicant will be requested to settle the grant and publication fees. Accordingly, the letters patent will be issued and the decisions to grant the patent will be published in the Patent Official Gazette for opposition purposes.
Opposition
Oppositions may be filed within 90 days from publication date before the Board of Grievances.
Protection Term
The term of protection is 20 years from filing date.
Annuities
A maintenance fee is due annually on patents and is payable the first 3 months of each calendar year following the year the patent application was filed. There is a 3-month grace period for late payment with a surcharge.
Compulsory Licensing
A patent has to be worked. If the patent is not being fully exploited by the patentee within 4 years from the date of filing or 3 years from the date of grant, the patent will be subject to compulsory licensing under the provisions of the law.
Naming of the Inventor on the Letters Patent
Compulsory
Employer and Employee’s Rights
Employer’s Rights
The employer shall be the patentee
(1) if the invention is made in execution of a contract or a commitment for the execution of inventive efforts, unless the work contract stipulates otherwise, or
(2) if the employee would not have developed the subject matter of the protection had he not used facilities, means or data made available through his employment
Employee’s Rights
The employee has the right to receive a remuneration to be agreed upon with the consent of both parties or assessed in light of the various circumstances of the contract of employment and the economic importance of the subject matter of the protection. Any special agreement depriving the employee of this right shall be null and void.
General Provisions
A patent application filed by the employee within two years from the date of termination of employment will be considered as if submitted during employment.
All previous provisions will apply to government employees.
Patent Linkage
Requests for marketing approval of generic drugs must include details on the corresponding patent if available (filing no., filing date, and country of grant). The SFDA will then contact the Saudi Patent Office to confirm whether a patent is involved before giving marketing approval. The GCC Patent Office is not usually contacted.
Filing Requirements
1. Power of attorney, legalized up to the Saudi Consulate
2. 2 copies of the specifications in English with Arabic translation
3. 2 sets of drawings in Arabic and one in English.
4. Deed of assignment from the inventor(s), legalized up to the Saudi Consulate
5. Copy of priority document, if priority is claimed, certified.
Item 3 must be submitted at the time of filing. Document 5 must be submitted within 12 months from priority date.
Documents 1 and 4 may be submitted within 1 month from filing date.
GCC Protection
Patent protection in Saudi Arabia can also be obtained through the Gulf Cooperation Council unified patent registration system (GCC patent law of 1999).
2011 Figures
Saudi Arabia ranked first among the Arab countries in the number of patents granted for the year 2011 which amounted to 147, based on WIPO sources. It also ranked first in the number of patents filed in the same year which amounted to 78.
For more specific legal questions relating to the Saudi Arabian patent system, please contact International_legal_affairs@epo.org.
There are two options for protecting an invention on Saudi Arabian territory:
All the information presented below relates to the first option, protection via the Saudi Arabian Patent Office.
Saudi Arabia has patents, plant varieties, industrial designs, layout designs of integrated circuits and trade marks, but not utility models. Patents and designs can be registered at the Saudi Arabian Patent Office (official name: General Directorate of Patents at King Abdulaziz City of Science and Technology (KACST)). Trade marks can be registered at the Ministry of Commerce and Industry.
The terms of protection for the different types of intellectual property are as follows:
 riyadh
riyadh
Article 4 of the Saudi Arabian Patent Law states the following:
“(a) The protection document shall not be granted if its commercial exploitation violates the Shari’ah (Islamic law).
(b) The protection document shall not be granted if its commercial exploitation is harmful to life, to human, animal or plant health, or is substantially harmful to the environment.”
Article 45 of the Saudi Arabian Patent Law states the following:
“In the application of provisions of this Law, the following shall not be regarded as inventions:
(a) Discoveries, scientific theories and mathematical methods.
(b) Schemes, rules and methods of conducting commercial activities, exercising pure mental activities or playing a game.
(c) Plants, animals and processes – which are mostly biological – used for the production of plants or animals, with the exception of micro-organisms, non-biological and microbiology processes.
(d) Methods of surgical or therapeutic treatment of human or animal body and methods of diagnosis applied to human or animal bodies, with the exception of products used in any of these methods.”
The exclusion also applies to computer programs and any other copyright work.
Computer programs as such are not patentable, but may be protected by copyright. Computer-related inventions may be patentable in Saudi Arabia if the requirements for patentability are met.
As of 3 August 2013, Saudi Arabia will be bound by the PCT. From that date onwards, nationals and residents of Saudi Arabia will be entitled to file international applications under the PCT.
All non-residents wishing to apply for a patent require an authorised Saudi Arabian representative. Applicants have to file a power of attorney which has been duly notarised and legalised by the consulate of Saudi Arabia.
More information:
Documents submitted under the Saudi Arabian Law of Patents must be in Arabic.
Yes, the priority term is 12 months from the earliest claimed priority, as stipulated in the Paris Convention. Saudi Arabia has been a member of the Paris Convention since 2004.
Yes. Where an application contains two or more inventions, applicants may submit a divisional application on their own initiative any time before the decision to grant or reject the application.
You do not need to file a request for examination. Applications are examined automatically, on the basis of the filing date. It is possible to withdraw applications.
Yes, you can withdraw an application before it is published.
No, it is not possible to request early publication of an application.
No, it is not possible to submit third-party observations.
 King Abdullah Economic City
King Abdullah Economic City
Responses to official actions should be submitted within 90 days from the date of the action.
Annual fees are due at the beginning of each year (1 January until 30 March), starting from the year following the filing date.
Applicants or patent holders who fail to pay within a maximum period of three months from the due date are liable to pay double the amount of the fee. If they fail to pay after being warned during the three months following the expiration of the first three months, the patent will cease to be valid and this will be recorded in the Register and published in the Gazette.
In case of force majeure it is possible to have a lapsed patent restored at the Saudi Arabian Patent Office.
No. In Saudi Arabia the 20-year term for patents cannot be extended. The Saudi Arabian Patent Law does not include any provisions on patent term extensions or supplementary protection certificates (SPCs).
Within 90 days from publication of the decision to grant, any interested party may apply for partial or total revocation of the patent.
Invalidation is possible for a third party at any time after grant and must be raised before a separate governmental body (Appeals Committee).
The Saudi Arabian Patent Office has an English-language database known as “IP search”. On this platform you can perform searches according to dates, numbers or applicant/inventor names.
If you require a more detailed answer, please send us your question using the contact form.
In general, the Saudi Arabian Patent Office does not produce any English abstracts for its patent documents. Where a non-Saudi Arabian applicant files an application, the abstract may also be in English (and in Arabic), while the rest of the patent has to be in Arabic only. Therefore, English abstracts are also available in certain circumstances.
There are no machine translations of Saudi Arabian patents available yet.
In general, legal-status information for Saudi Arabian patents is not yet available, but a brief “application status” can be found together with the bibliographic information in the “IP search” database.
Information about fees is currently available in Arabic only.
The KACST website gives detailed information about the role of patent attorneys and the services they provide. It is available in Arabic only.
If you require a more detailed answer, please send us your question using the contact form.